Forskere, der arbejder i syntetisk organisk kemi, er under stigende pres for at opdage og udvikle innovative syntetiske forløb og robuste kemiske processer så hurtigt som muligt. In-line procesanalytiske teknologier (PAT) er i stand til at levere vigtige spor, der sætter forskere i stand til at forstå kinetik, forløb, og mekanismer i kemiske reaktioner. Bevæbnet med øget forståelse for reaktioner, er forskerne i stand til hurtigt at optimere og optrappe processer med øget robusthed og ydeevne. Se applikationsbiblioteket for de seneste udgivelser og industrielle applikationer.

Forbedring af katalysatorydeevne af
Tandem-hydroformylation/hydrogenering
Forskere anvendte in situ reaktionsovervågning for at forstå aktivitet og robusthed af nye komponenter i hydroformylering/hydrogeneringskatalysator. Ved at måle kinetik, forløb og reaktionsmekanismer, blev der identificeret de optimale betingelser for katalysatorydeevne.

Reaktionsovervågning i realtid
Da det passer til en bred vifte af kemiske anvendelser, giver in situ Fourier Transform Infrarød (FTIR) spektroskopi overvågning i realtid af centrale reaktionstyper. Da det er designet til at følge reaktionsforløbet, giver ReactIR specifikke oplysninger om igangsættelse af reaktioner, konvertering, mellemprodukter og slutpunkt. Dette sker selv under vanskelige forhold, der gør offline prøvetagning og analyse vanskelig, såsom reaktioner under tryk eller ekstreme temperaturer. Applikationer omfatter Katalysatoroptimering, Hydrogenering, Polymerisering og meget reaktiv kemi.
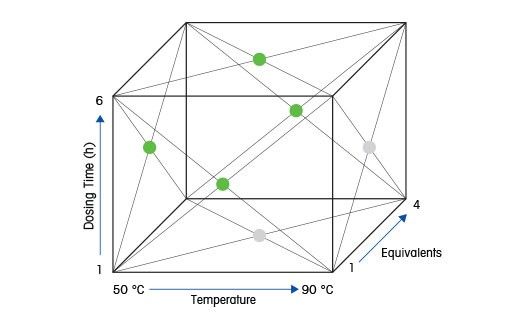
Design af forsøg (DoE)
til optimerede reaktionsbetingelser
Forskere anvender ofte Design af forsøg (DoE) til maksimal information, når de planlægger kontrollerede forsøg. Produktsammensætning, stereospecificitet, udbytte og urenheder optimeres ved at ændre reaktionsbetingelserne, såsom temperatur, opløsningsmiddel, katalysator, og koncentrationer af substrat eller reagens. En effektiv undersøgelse af de påvirkende faktorer med kun et lille antal eksperimenter, kræver at eksperimenterne udføres under velkontrollerede, nøjagtige og reproducerbare betingelser. Alt dette finder fortrinsvis sted automatiseret eller semi-automatiseret i lille målestok og kan hurtigt føre til optimerede reaktionsbetingelser.

Arbejdsstationer til organisk syntese
Nye teknikker til bedre kemi
Arbejdsstationer til organisk syntesemed lille volumen gør det muligt for kemikere hurtigt og effektivt at udføre eksperimenter dag og nat med kontrol over temperatur, blanding, dosering og pH. Kombinationen af automatiserede laboratoriereaktorer med uovervåget repræsentativ prøvetagning eller in situ analyseværktøjer giver et ekstra niveau af forståelse for procesudvikling, fra partikelstørrelse til det molekylære niveau af reaktionsforløb, kinetik og reaktionsprogression. Disse arbejdsstationer til organisk syntese er nemme at bruge, meget gentagelige og forbundet med hinanden via softwarekontrol og datadeling.

Innovative teknikker
Til at syntetisere gennembrud for molekyler
Opdag, hvordan forskerne anvender effektive metoder til at udvikle nye syntetiske baner og optimere kritiske procesforhold. Fire casestudier belyser, hvordan førende farmaceutiske virksomheder udvider synteselaboratorieydeevne.

Opskalering og optimering
Meget reaktiv kemi
Meget reaktiv kemi har potentielt farlige reaktanter, mellemprodukter og produkter og involverer ofte meget eksotermiske reaktioner. Sikring af sikre driftsforhold, minimering af menneskelig eksponering og opnåelse af den maksimale mængde information i hvert forsøg er nøglefaktorer i succesfuldt design af og optrapning af meget reaktive kemier. In situ-reaktionsovervågning er kritisk, da meget reaktive materialer ofte er ustabile, hvilket begrænser offline prøvetagning. Eksempler omfatter syntese af Grignard-reagenser.
Applikationer
Applikation af syntetisk organisk kemi
Isocyanates are critical building blocks for high performance polyurethane-based polymers that make up coatings, foams, adhesives, elastomers, and insulation. Concerns over exposure to residual isocyanates led to new limits for residual isocyanates in new products. Traditional analytical methods for measuring the residual isocyanate (NCO) concentration using offline sampling and analysis raise concerns. In situ monitoring with process analytical technology addresses these challenges and enables manufacturers and formulators to ensure that product quality specifications, personnel safety, and environmental regulations are met.
Polymerization reaction measurement is crucial to produce material that meets requirements, including Immediate understanding, accurate and reproducible, Improved safety.
Impurity profiling aims at identification and subsequent quantification of specific components present at low levels, usually less than 1% and ideally lower than 0.1 %.
Grignard reactions are one of the most important reaction classes in organic chemistry. Grignard reactions are useful for forming carbon-carbon bonds. Grignard reactions form alcohols from ketones and aldehydes, as well as react with other chemicals to form a myriad of useful compounds. Grignard reactions are performed using a Grignard reagent, which is typically a alkyl-, aryl- or vinyl- organomagnesium halide compound. To ensure optimization and safety of Grignard reactions in research, development and production, in situ monitoring and understanding reaction heat flow is important.
Hydrogenation reactions are used in the manufacturing of both bulk and fine chemicals for reducing multiple bonds to single bonds. Catalysts are typically used to promote these reactions and reaction temperature, pressure, substrate loading, catalyst loading, and agitation rate all effect hydrogen gas uptake and overall reaction performance. Thorough understanding of this energetic reaction is important and PAT technology in support of HPLC analysis ensure safe, optimized and well-characterized chemistry.
Highly reactive chemistry is a terminology used to describe chemical reactions that are particularly challenging to handle and develop due to the potentially hazardous and/or energetic nature of the reactants, intermediates and products that are present during synthesis. These chemistries often involve highly exothermic reactions which require specialized equipment or extreme operating conditions (such as low temperature) to ensure adequate control. Ensuring safe operating conditions, minimizing human exposure, and gaining the maximum amount of information from each experiment are key factors in successfully designing and scaling-up highly reactive chemistries.
Many processes require reactions to be run under high pressure. Working under pressure is challenging and collecting samples for offline analysis is difficult and time consuming. A change in pressure could affect reaction rate, conversion and mechanism as well as other process parameters plus sensitivity to oxygen, water, and associated safety issues are common problems.
Halogenation occurs when one of more fluorine, chlorine, bromine, or iodine atoms replace one or more hydrogen atoms in an organic compound. Depending on the specific halogen, the nature of the substrate molecule and overall reaction conditions, halogenation reactions can be very energetic and follow different pathways. For this reason, understanding these reactions from a kinetics and thermodynamic perspective is critical to ensuring yield, quality and safety of the process.
Catalysts create an alternative path to increase the speed and outcome of a reaction, so a thorough understanding of the reaction kinetics is important. Not only does that provide information about the rate of the reaction, but also provides insight into the mechanism of the reaction. There are two types of catalytic reactions: heterogeneous and homogeneous. Heterogeneous is when the catalyst and reactant exist in two different phases. Homogeneous is when the catalyst and the reactant are in the same phase..
En syntesereaktion er en kemisk proces, hvor enkle elementer eller forbindelser kombineres for at danne et mere komplekst produkt. Det er repræsenteret af ligningen: A + B → AB.
Design of Experiments (DoE) requires experiments to be conducted under well-controlled and reproducible conditions in chemical process optimization. Chemical synthesis reactors are designed to perform DoE investigations ensuring high quality data.
Reaction mechanisms describe the successive steps at the molecular level that take place in a chemical reaction. Reaction mechanisms cannot be proven, but rather postulated based on empirical experimentation and deduction. In situ FTIR spectroscopy provides information to support reaction mechanisms hypotheses.
Organometallic Synthesis, or Organometallic Chemistry, refers to the process of creating organometallic compounds, and is among the most researched areas in chemistry. Organometallic compounds are frequently used in fine chemical syntheses and to catalyze reactions. In situ Infrared and Raman spectroscopy are among the most powerful analytical methods for the study of organometallic compounds and syntheses.
Oligonucleotide synthesis is the chemical process by which nucleotides are specifically linked to form a product of desired sequenced.
Alkylation is the process by when an alkyl group is added to a substrate molecule. Alkylation reactions are a widely used technique in organic chemistry.
This page outlines what epoxides are, how they are synthesized and technology to track reaction progression, including kinetics and key mechanisms.
The Suzuki and related cross-coupling reactions use transition metal catalysts, such as palladium complexes, to form C-C bonds between alkyl and aryl halides with various organic compounds.
Lithiation and organolithium reactions are key in the development of complex pharmaceutical compounds; organolithium compounds also act as initiators in certain polymerization reactions.
C-H bond activation is a series of mechanistic processes by which stable carbon-hydrogen bonds in organic compounds are cleaved.
Organocatalysis refers to the employment of particular organic molecules to speed up chemical reactions through catalytic activation.
Hydroformylation, also known as oxo synthesis, is a chemical reaction involving the addition of carbon monoxide (CO) and hydrogen (H₂) to an unsaturated compound, typically an alkene or alkyne. The reaction is catalyzed by transition metal complexes, such as rhodium or cobalt, leading to the formation of aldehydes or aldehyde derivatives.
Click reactions refer to chemical reactions that meet the criteria of click chemistry. Click reactions are typically fast, high-yielding, and occur under mild conditions, making them ideal for a variety of applications.
En kontinuerlig omrørt tankreaktor (CSTR) er en beholder, hvor reagenser og reaktanter strømmer ind i en reaktor, mens reaktionsproduktet kommer ud af beholderen.
Isocyanates are critical building blocks for high performance polyurethane-based polymers that make up coatings, foams, adhesives, elastomers, and insulation. Concerns over exposure to residual isocyanates led to new limits for residual isocyanates in new products. Traditional analytical methods for measuring the residual isocyanate (NCO) concentration using offline sampling and analysis raise concerns. In situ monitoring with process analytical technology addresses these challenges and enables manufacturers and formulators to ensure that product quality specifications, personnel safety, and environmental regulations are met.
Grignard reactions are one of the most important reaction classes in organic chemistry. Grignard reactions are useful for forming carbon-carbon bonds. Grignard reactions form alcohols from ketones and aldehydes, as well as react with other chemicals to form a myriad of useful compounds. Grignard reactions are performed using a Grignard reagent, which is typically a alkyl-, aryl- or vinyl- organomagnesium halide compound. To ensure optimization and safety of Grignard reactions in research, development and production, in situ monitoring and understanding reaction heat flow is important.
Hydrogenation reactions are used in the manufacturing of both bulk and fine chemicals for reducing multiple bonds to single bonds. Catalysts are typically used to promote these reactions and reaction temperature, pressure, substrate loading, catalyst loading, and agitation rate all effect hydrogen gas uptake and overall reaction performance. Thorough understanding of this energetic reaction is important and PAT technology in support of HPLC analysis ensure safe, optimized and well-characterized chemistry.
Highly reactive chemistry is a terminology used to describe chemical reactions that are particularly challenging to handle and develop due to the potentially hazardous and/or energetic nature of the reactants, intermediates and products that are present during synthesis. These chemistries often involve highly exothermic reactions which require specialized equipment or extreme operating conditions (such as low temperature) to ensure adequate control. Ensuring safe operating conditions, minimizing human exposure, and gaining the maximum amount of information from each experiment are key factors in successfully designing and scaling-up highly reactive chemistries.
Many processes require reactions to be run under high pressure. Working under pressure is challenging and collecting samples for offline analysis is difficult and time consuming. A change in pressure could affect reaction rate, conversion and mechanism as well as other process parameters plus sensitivity to oxygen, water, and associated safety issues are common problems.
Halogenation occurs when one of more fluorine, chlorine, bromine, or iodine atoms replace one or more hydrogen atoms in an organic compound. Depending on the specific halogen, the nature of the substrate molecule and overall reaction conditions, halogenation reactions can be very energetic and follow different pathways. For this reason, understanding these reactions from a kinetics and thermodynamic perspective is critical to ensuring yield, quality and safety of the process.
Catalysts create an alternative path to increase the speed and outcome of a reaction, so a thorough understanding of the reaction kinetics is important. Not only does that provide information about the rate of the reaction, but also provides insight into the mechanism of the reaction. There are two types of catalytic reactions: heterogeneous and homogeneous. Heterogeneous is when the catalyst and reactant exist in two different phases. Homogeneous is when the catalyst and the reactant are in the same phase..
Reaction mechanisms describe the successive steps at the molecular level that take place in a chemical reaction. Reaction mechanisms cannot be proven, but rather postulated based on empirical experimentation and deduction. In situ FTIR spectroscopy provides information to support reaction mechanisms hypotheses.
Organometallic Synthesis, or Organometallic Chemistry, refers to the process of creating organometallic compounds, and is among the most researched areas in chemistry. Organometallic compounds are frequently used in fine chemical syntheses and to catalyze reactions. In situ Infrared and Raman spectroscopy are among the most powerful analytical methods for the study of organometallic compounds and syntheses.
Hydroformylation, also known as oxo synthesis, is a chemical reaction involving the addition of carbon monoxide (CO) and hydrogen (H₂) to an unsaturated compound, typically an alkene or alkyne. The reaction is catalyzed by transition metal complexes, such as rhodium or cobalt, leading to the formation of aldehydes or aldehyde derivatives.