Guide to Crystallization Development
Seeding a Crystallization
To Control Crystal Size
Seeding is one of the most straightforward methods used to control supersaturation. During seeding, a small mass of crystals is added to a supersaturation in order to:
- Start the crystallization at the desired supersaturation level
- Provide sufficient surface area to ensure supersaturation is consumed in a controlled way
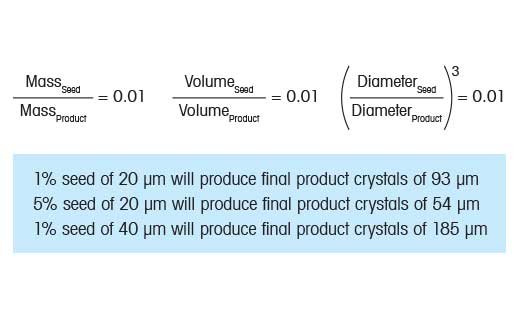
Choosing the correct seed loading (mass) and seed size can helop produce final crystal product of a specified size. If we consider a theoretical crystallization system where only growth occues and the crystals are spherical, it is possible to develop a simple model where the final crystal size can be predicted simply based on the starting seed size and loading (right). Consider the case where we seed a crystallization with 1% seed. In this case, 1% is simply the ration of seed mass to the final anticipated product mass. Since the seed and final product have identical density, it is simple to convert mass ratio to volume ration. Then, the next logical step is to convert volume ratio to diameter ratio.
Crystal Size and Shape
Dendritic Growth
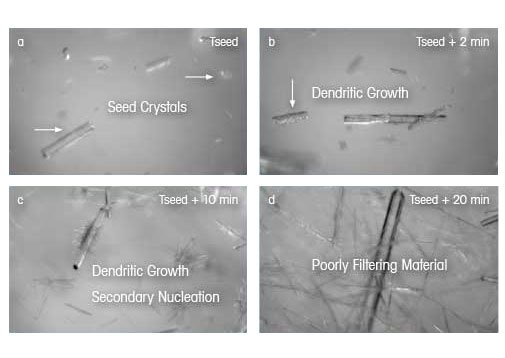
While this simple model is useful for demonstrating how seed size and loading affect the final crystal size distribution, the assumptions are not commonly observed in real systems. Crystals are rarely spherical, meaning more complex models are needed to predict the size of needles. Crystallization processes are rarely, if ever, completely growth dominated. Some degree of nucleation and attrition almost always occurs in order to develop an effective seeded crystallization. As this example demonstrates, real-time microscopy offers a unique opportunity to better understand seeding events. In the images on the right, the seeding process is observed directly during an organic crystallization using real time microscopy. After seed crystals are added to the supersaturated solution (a), it becomes apparent that the surface nucleation on the seed crystals occurs (b). Over time, dendritic growth occurs with small crystal "branches" growing orthogonally from the seed crystal (c). After thirty minutes, a bimodal size and shape distribution is present, indicating that the final crystal product may filter and dry poorly (d).

Real-Life Application Examples
Visualizing Seeding Mechanisms
Process knowledge can be easily obtained by visualizing seeding mechanisms during crystallization development.
Choosing a Seeding Temperature
The Effect on Crystallization Kinetics
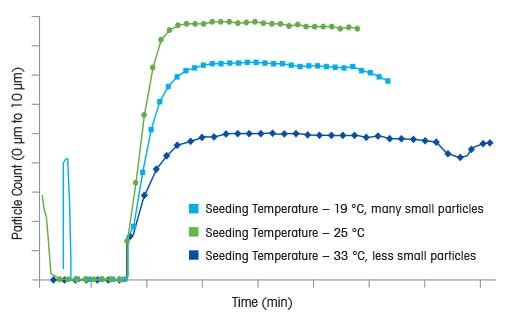
The supersaturation level at which seed will be added is another critical variable to consider when designing a seeded crystallization process. In a cooling crystallization, this might be referred to as the “seeding temperature”, but it is actually the supersaturation level that is being considered. Seeding at high supersaturation levels may result in excessive secondary nucleation, rendering the seeding process itself redundant, unless the goal is a fine crystal size distribution. If crystal growth is desired, then seeding closer to the solubility curve, at lower supersaturation, may be a wise choice. This approach is shown in the graph to the right, where three crystallization processes are compared using ParticleTrack with FBRM technology at three different seeding temperatures. By comparing particle counts between 0 μm and 10 μm for each crystallization, it is possible to compare relative nucleation rates at different seeding temperatures. The lowest seeding temperature (highest supersaturation) results in the highest degree of nucleation and fine crystals at the end of the process.
Seed Dispersion
Tracking Seed Size and Count Over Time
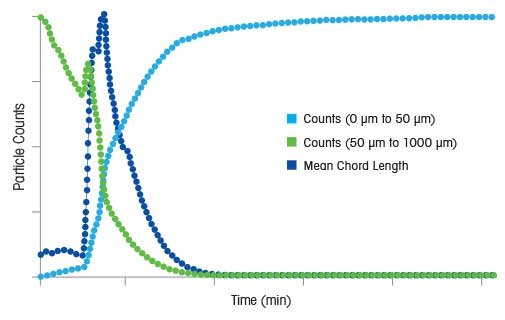
When seeding, another important factor to consider is that during preparation and storage, seed crystals can stick together and form aggregates. Often, an isothermal hold after seeding is required to ensure that seed crystals are able to fully disperse, and the full surface area is available for crystallization to progress. Such an isothermal hold can also help seed crystals grow, increasing the surface area available for growth. In the example on the right, a ParticleTrack process trend that describes a crystallization process where it takes four hours for seeds to fully disperse. This example, along with the others provided above, indicate that careful characterization of the seeding process, in terms of a number of critical process variables, is vital to ensure consistency and product quality.

Seeding a Crystallization Process
Although crystallization has improved over the years, the seeding step still presents challenges. This paper reviews how to design a seeding strategy and what parameters should be considered when implementing a seeing protocol.
Technologies to Monitor, Optimize, and Control
Crystallization unit operations offer the unique opportunity to target and control an optimized crystal size and shape distribution to:
- Reduce Filtration and Drying Times
- Avoid Storage, Transport and Shelf Life Issues
- Ensure a Consistent and Repeatable Process at Lower Costs

















