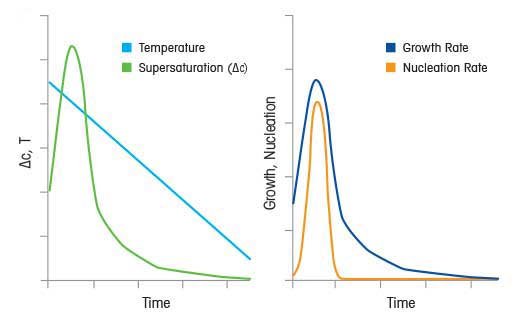
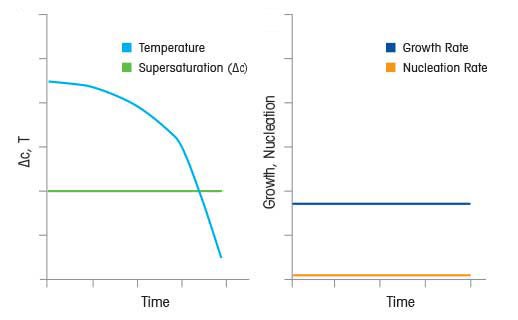
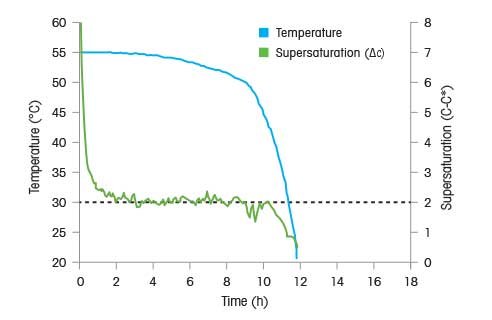
Crystallization kinetics are characterized in terms of two dominant processes, nucleation kinetics and growth kinetics, occurring during crystallization from solution.
- Nucleation Kinetics - the rate of formation of a stable nuclei
- Growth Kinetics - the rate at which a stable nuclei grows to a macroscopic crystal.