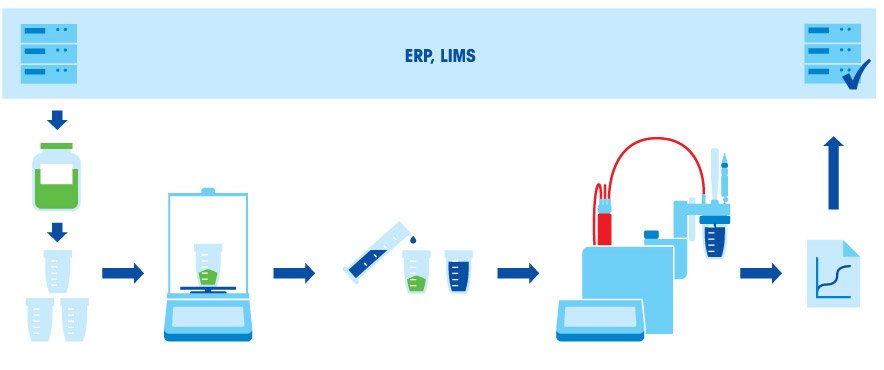
Titration is a widely used analytical technique. It can be used for the analysis and quality control of products across industries such as Pharmaceuticals, Chemicals, Petrochemicals, Food and Beverages etc. Several parameters can be determined, for example: Acidity, alkalinity, metal ions, and active pharmaceutical ingredients. For determination of water content, a special type of titration, known as Karl Fischer Titration, is used.
Titration is the determination of the quantity of a specific substance (analyte) contained in a sample by the controlled addition of a reagent (titrant) of known concentration based on the complete chemical reaction between the substance and the reagent. The titrant is added until the reaction is complete (equivalence point).
The classical way of monitoring a titration reaction is by adding a suitable indicator to the analyte which changes color when the chemical reaction is complete (titration end-point). Today, the chemical reaction and the end-point can be monitored by means of a sensor.
Why Accurate Weighing is Crucial
From the consumption and concentration of the titrant as well as the weight of the sample used in the analysis, the content of the analyte can be calculated. Accurately weighing the substances used to prepare both the titrant solution and the analyte sample solution is highly important. Only with accurate sample preparation you can be sure of accurate titration results.
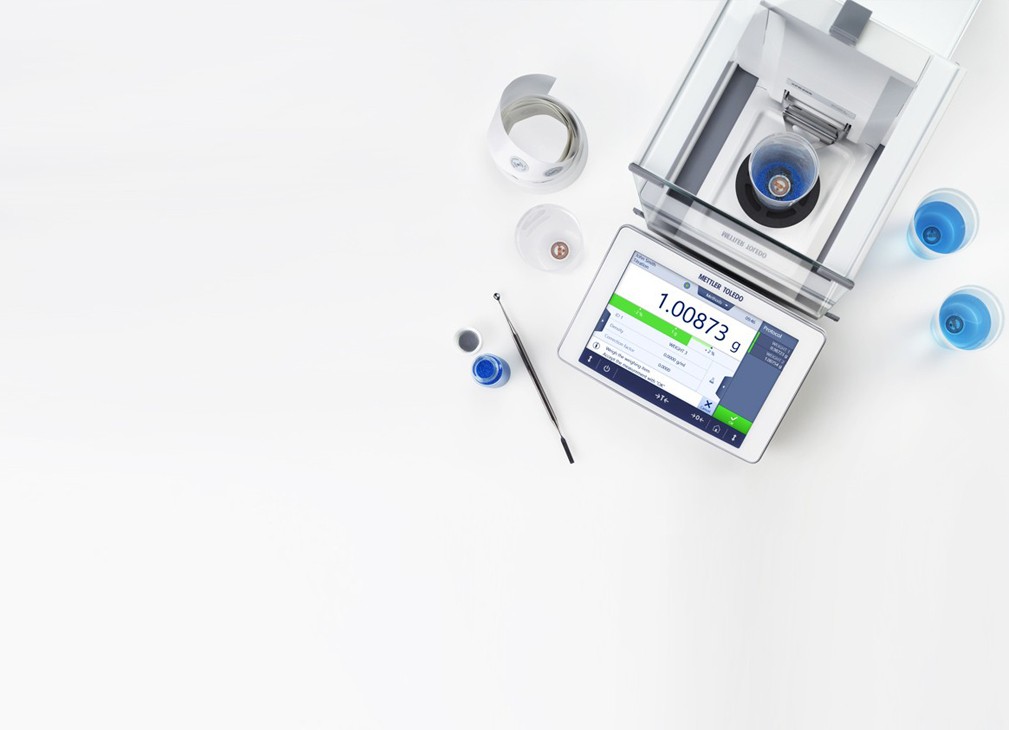
The SmartSample™ weighing system for titration automation increases sample identification and efficiency with RFID technology.