 |
Table of Contents:
TA Tip
- Curve interpretation Part 4: TGA measurements
News
- STARe Software V14.0
- DSC 3 and DSC 3+
- TGA 2 and TGA/DSC 3+
- Accurate measurements with GWP® − the global weighing standard
Applications
- Reliable determination of sample mass − the concept of minimum weight
- Characterization of shape memory alloys by DSC and DMA, Part 2: DMA analysis
- Curing behavior of aqueous resins using high pressure crucibles
- Separation of melting and decomposition using high heating rates
Reliable determination of sample mass – the concept of minimum sample weight
In thermal analysis, the accurate weighing of samples in the milligram range is usually the first step of the actual experiment, especially for DSC and TGA measurements. The accuracy with which the initial sample mass can be determined has a direct effect on the accuracy of the measurement results (for example the specific enthalpy in DSC or the content in TGA). In this article, we discuss the basic concepts that prevent errors in the determination of sample mass and that ensure that requirements regarding accuracy are adhered to. These concepts are based on GWP®, the science-based global standard for the efficient lifecycle management of weighing instruments.
Introduction
There are several factors that limit the performance of a balance with regard to the accuracy with which the mass of a sample can be determined. Besides the finite readability due to rounding of a digital indication, the most important of these are repeatability (RP), eccentricity (EC), nonlinearity (NL), and sensitivity (SE). These terms are graphically displayed in Figure 1 and explained in detail in the respective technical literature [1].
[…]
References
[1] R. Nater, A. Reichmuth, R. Schwartz, M. Borys and P. Zervos, Dictionary of Weighing Terms - A Guide to the Terminology of Weighing, Springer, 2009.
Characterization of shape memory alloys by DSC and DMA, Part 2: DMA analysis
In Part 1 of this series, we studied the properties of nitinol using differential scanning calorimetry (DSC). In this second part, we investigate the behavior of nitinol using dynamic mechanical analysis (DMA).
Introduction
The deformation of an object made from a shape memory alloy such as nitinol can be completely reversed either by heating (shape memory effect) or by elimination of the stress that produced the deformation (superelasticity). These unusual properties are a consequence of reversible transformation of the crystal lattice.
Part 1 of this series [1]) dealt with the thermally induced transformation of the crystal lattice. We used DSC measurements to investigate the shape memory effect using nitinol as an example.
This second part describes the superelastic behavior of nitinol and shows how dynamic mechanical analysis (DMA) can be used to study this effect.
The DMA experiments were carried out in the tension mode using DMA/SDTA861e and DMA 1 instruments.
[…]
References
[1] N. Fedelich, Characterization of shape memory alloys by DSC and DMA, Part 1: Characterization by DSC, UserCom 40, 10–14.
Curing behavior of aqueous resins using high pressure crucibles
The curing behavior of an aqueous melamine-formaldehyde resin was investigated by DSC. Predictions were then made for the curing reaction at different isothermal temperatures using model free kinetics (MFK). The validity of the predictions was checked and confirmed by practical isothermal measurements.
Introduction
Melamine-formaldehyde resins are aqueous liquids that are used for making decorative surfaces on wood products and for coating laminates. The resins release water during the curing or so-called condensation reaction.
Optimum curing of the resins is crucial for ensuring that the final products exhibit perfect surface properties. This presents a challenging development task [1]. DSC investigations under optimized conditions followed by kinetic predictions can be very helpful for this work [2 , 3].
[…]
References
[1] Technical Conference Management- European Laminates Conference and Workshop 2010, A. Kandelbauer, G. Wuzella, A. R. Mahendran, Potential of advanced analysis of thermochemical data for predicting technological properties of decorative surfaces
[2] Model free kinetics, UserCom 2, 7.
[3] Tips on model free kinetics, UserCom 8, 1–3.
Separation of melting and decomposition using high heating rates
Many organic materials are chemically stable in the solid form but decompose when melting begins. In such cases, the separation of melting effects and the decomposition reaction is not possible by conventional DSC. In this article, we show how the two effects can however be separated using the Flash DSC 1 at high heating rates. The melting process and decomposition reaction can then be individually evaluated.
Introduction
A lot of information about organic substances can be derived from the melting peak in a DSC measurement. This includes the degree of purity, composition, polymorphic effects, the enthalpy of melting and the melting point itself. The information can only be obtained with adequate accuracy if the sample is chemically stable during the melting process.
Many organic materials, however, start to decompose as soon as melting begins. As a result, the DSC curve exhibits two overlapping thermal events that cannot easily be separated. Furthermore, decomposition leads to contamination of the sample and in turn to a change in the measured melting point [1].
One possibility to separate melting and decomposition is to measure the sample at a high heating rate. This approach takes advantage of the effect that the temperature of the melting process is not (or is only slightly) dependent on the heating rate whereas a chemical reaction is shifted to higher temperatures at higher heating rates.
If the heating rate is sufficiently high, it should therefore be possible to shift a decomposition reaction to a temperature high enough to separate the processes of melting and decomposition. Measurements using conventional DSC, however, show that the technical limit of the DSC is frequently reached before the two processes can in fact be separated.
Figure 1 shows an example of an organic pigment that was measured at heating rates of 100 and 150 K/min. In both curves, it is apparent that melting and decomposition overlap; the thermal events cannot be correctly evaluated in either of the curves. Much higher heating rates are therefore necessary to separate the two effects. Such rates can now be achieved using the METTLER TOLEDO Flash DSC 1 [2–4], which can reach heating rates of up to 2.4 million K/min or 40,000 K/s.
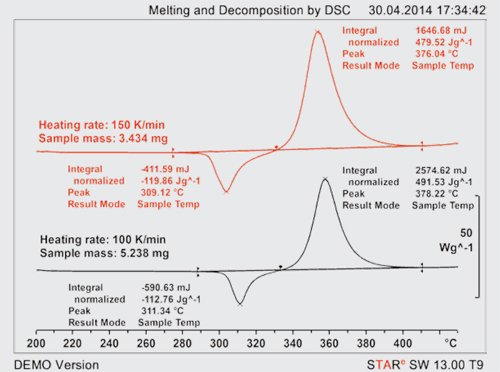 |
Figure 1. Conventional DSC measurements at heating rates of 100 and 150 K/min. |
In this article, Flash DSC 1 measurements are shown in which the melting and decomposition effects of an organic substance are separated and individually analyzed.
[…]
References
[1] R. Riesen, Influence of the heating rate: Melting and chemical reactions, UserCom 23, 20–22.
[2] The new Flash DSC 1, UserCom 32, 6 –7.
[3] J. Schawe, Practical aspects of the Flash DSC 1: Sample preparation for measurements of polymers, UserCom 36, 17–24.
[4] J.E.K. Schawe, Influence of processing conditions on polymer crystallization measured by fast scanning DSC, J. Therm. Anal. Calorim. (2013).




