 |
Table of Contents:
TA Tip
- Thermal analysis of polymers; Part 1: DSC of thermoplastics
New in our sales program
- The new comprehensive thermal analysis handbook
- Melting point software, Version 1.1
Applications
- Curing kinetics of EVA using DSC, DMA and model free kinetics
- UV curing of a cycloaliphatic epoxy resin using TOPEM® and conventional DSC
- Analysis of the components of a sandwich composite panel by DMA
- TGA/MaxRes used for studies on hydrogen storage
Curing kinetics of EVA using DSC, DMA and model free kinetics
The use of solar panels is well-known for converting sunlight to electricity. This so-called photovoltaic electrical power is expected to make an important contribution to providing a sustainable supply of energy in the future.
Introduction
A photovoltaic module consists of arrays of jointly connected solar cells. An important step in the manufacture of a photovoltaic module is encapsulation. In this production step, solar cells are encapsulated between a glass sheet and a Tedlar film as backing sheet.
Encapsulation is commonly performed using a 0.4-mm thick ethylene-vinyl acetate (EVA) film. It seals the module and protects it against environmental influences such as moisture, oxygen, and weathering. This is very important because a guaranteed lifetime of 25 years is nowadays usual. In this article, we show how DSC and DMA experiments followed by evaluation with model free kinetics were used to investigate the curing behavior of EVA during the lamination process. Studies like this allow the optimum lamination conditions to be determined; the results can also be used for quality control.
In modern photovoltaic modules, the solar cells are encapsulated between a glass sheet and a backing sheet, usually a Tedlar® film [1, 2, 3], as illustrated in Figure 1. Sheets of ethylene-vinyl acetate (EVA) are placed between the solar cells and the backing sheet and the glass. In the production process, the sandwich is pressed into place and heated. The EVA cures and provides a permanent and tight seal.
EVA has many excellent long-term properties such as its optical transmittance in the visible region, chemical resistance toward UV light, and electrical insulation. EVA is a block copolymer and in this application typically consists of 67% polyethylene and 33% vinyl acetate (see Figure 2). Uncured EVA is a thermoplastic material that on heating first exhibits a glass transition and then a melting process. In the curing process, the EVA chains undergo crosslinking. The curing reaction is initiated by a peroxide compound. This decomposes on heating and splits into two oxyradicals that promote the crosslinking of the EVA polymer. EVA only becomes mechanically and chemically resistant at the high temperatures that occur in photovoltaic modules (up to 80 °C) after this curing process.
It is important to determine the degree of cure of the EVA in order to optimize the lamination conditions and control the quality control of the finished photovoltaic modules. In the past, this was done using a method based on solvent extraction. Recently it was shown that DSC measurements can also be used [4].
In this article, we show how the curing reaction can be described by model free kinetics using data from DSC and DMA measurements. This allows the lamination process to be optimized with regard to temperature and lamination time.
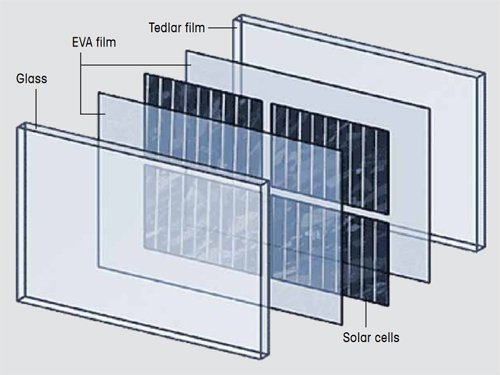 |
Figure 1. Construction of a photovoltaic module. |
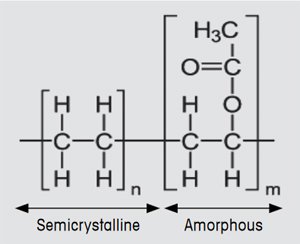 |
Figure 2. Chemical structure of EVA. |
[…]
References
[1] Michael DeBergalis, Journal of Fluorine Chemistry 125 (2004), 1255–1257.
[2] T. Krieger, H. Roekens-Guibert, Environmental impacts of Tedlar ® PVF film for use in photovoltaic modules, DuPont.
[3] A. K. Plessing, in: G. M. Wallner, R.W. Lang (Eds.), Proceedings of Polymeric Solar Materials, Leoben, Austria, 2003, pp. XII1–XII8.
[4] Z. Xia, D.W. Cunningham, J.H. Wohlgemuth, PV Modules, 5, 1(2009), 150–159.
UV curing of a cycloaliphatic epoxy resin using TOPEM® and conventional DSC
The curing of materials with (ultraviolet) light is often performed at relatively low temperatures, for example even at room temperature. Under these conditions, the material can vitrify. Depending on the temperature, curing slowly continues in the glassy state. The article shows how these processes can be investigated using TOPEM® and conventional DSC measurements.
Introduction
UV-curing paints and varnishes, coatings, and adhesives are nowadays widely used. The main practical advantages of such systems are that they cure within a few seconds and produce high quality coatings at relatively low temperatures (even at room temperature) so that the substrate is subjected to only low levels of thermal stress. In addition, there are important ecological advantages, for example no solvent emission and no drying processes with consequent energy consumption.
Difficulties arise only with the curing of pigmented paints and varnishes because the pigments also absorb UV light, which can lead to incomplete curing.
In this article, we show how isothermal curing processes of light-curing resins can be measured by DSC. We investigated the influence of temperature on the degree of cure determined from dynamic postcuring experiments. Furthermore, using TOPEM®, we describe the behavior of the resin immediately after exposure to the UV light.
[…]
Analysis of the components of a sandwich composite panel by DMA
The mechanical properties of the individual components of a sandwich composite panel were investigated by DMA. The temperature-dependent shear moduli of the polyurethane foam core and the bending modulus of the carbon fiber epoxy composite skin sheets are important quantities that determine the mechanical behavior of the sandwich construction.
Introduction
A sandwich composite panel consists of a lightweight core sandwiched between two thin, stiff skin sheets. Sandwich structures of this type are often employed when products have to exhibit high bending strength and stiffness but at the same time need to be light in weight. Typical examples of successful industrial applications are the wing flaps of aircraft, wind turbine blades, surfboards, and boat superstructures.
[…]
TGA/MaxRes used for studies on hydrogen storage
The production of energy from renewable sources is usually subject to fluctuations and requires intermediate storage systems. Hydrogen is a particularly promising possibility. One field of research is focused on developing ways to make hydrogen safe to use by storing it in suitable solids. Borohydrides show great potential. Hydrogen can be regenerated through controlled reaction with water. This produces hydrates of different composition. The following article shows how TGA can be used to study the stability ranges of the hydrates. This information is important for optimum process control and for obtaining a high yield of hydrogen.
Introduction
Renewable energies such as solar energy, wind energy or biomass are crucial to solving the energy crisis and providing a sustainable supply of energy.
A major difficulty with renewable forms of energy is that they are not continuously available (e.g. the day and night cycle with solar energy). To bridge gaps in supply, renewable energy forms have to be somehow temporarily stored. Hydrogen is a particularly promising candidate for this purpose. It can be generated electrolytically from water and converted to electricity as needed in a fuel cell thereby producing water once again. Hydrogen is thus a form of energy that can be stored and transported.
Hydrogen can in fact be stored and transported as a gas, as a liquid or bound in a solid. Due to safety regulations, its storage as a gas or liquid is rather difficult and expensive.
For this reason, research is focused on the storage of hydrogen in solids, for example as metal or complex hydrides [1]. The latter includes sodium borohydride, NaBH4 . This material can store up to 10.6 mass% hydrogen. Hydrogen is regenerated from the hydride through the controlled addition of water according to the following equation [2, 3]:
NaBH4 + (2 + x) H2O → NaBO2 ⋅ x H2O + 4 H2 where x = 0, 2, 4
The percentage yield of hydrogen depends on the degree of hydration x of the metaborate produced. For example, the hydrates bind additional water molecules that are then no longer available to generate hydrogen. The maximum hydrogen capacity is 10.6% if the anhydride is formed (x = 0), but only 5.5% if the tetrahydrate (x = 4) is formed. These calculations refer to the hydrogen that is produced from the sum of the reactants (sodium borohydride and water, see Table 1). For mobile applications in particular, the weight of water that has to be transported is important.
The aim of our research is to determine the different hydrate steps of the metaborate using thermogravimetric analysis (TGA) and in particular the MaxRes method. This information is important for defining the experimental conditions to ensure that only hydrates with relatively low degrees of hydration occur when hydrogen is generated from the sodium borohydride.
[…]
References
[1] A. Züttel, Hydrogen storage methods, Naturwissenschaften, 91 (2004) 157.
[2] H. I. Schlesinger, H. C. Brown, A. E. Finholt, J. R. Gilbreath, H. R. Hoekstra and E. K. Hyde, Sodium borohydride, its hydrolysis and its use as a reducing agent and in the generation of hydrogen, J. Am. Chem. Soc., 5 (1953) 215.
[3] R. Bouaziz, Contribution à l›étude des borates de lithium et de sodi - um, Ann. chim. (Paris), 6 (1961) 348–93.




