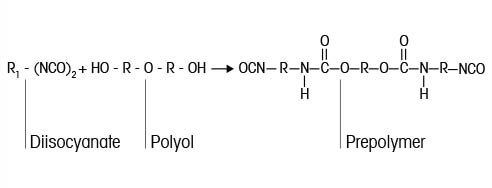
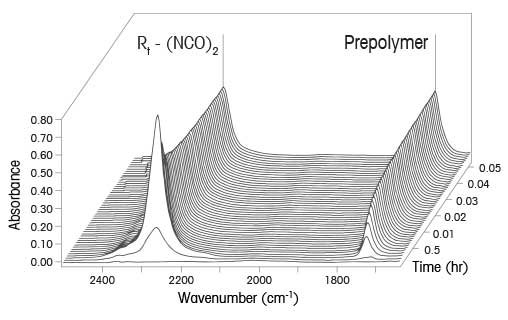
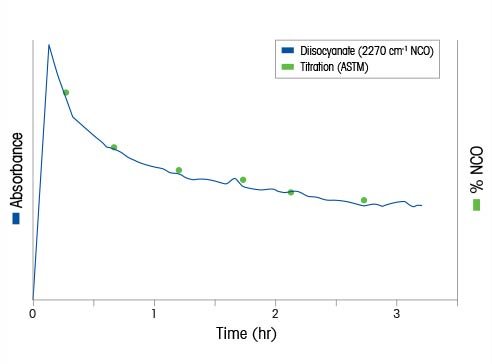
Isocyanates reactions are critical building blocks for high performance polyurethane-based polymers that make up coatings, foams, adhesives, elastomers and insulation. Concerns over exposure to residual isocyanates led to new limits for residual isocyanates in new products. Traditional analytical reaction methods for measuring the residual isocyanate (NCO) concentration using offline sampling and analysis raise concerns with:
- Long wait times for offline analytical results, making it impossible to make real-time decisions, leading to inconsistent product quality and reduction in production capacity
- Sufficient process knowledge lacking at any given time point
- Exposure to NCO samples increasing the risk of sensitization and human health risks