 |
This white paper details the regulatory requirements for computerized systems – such as thermal analyzers – and important steps to avoid costly data integrity violations. |
“Data integrity is the degree to which data are complete, consistent, accurate, trustworthy, reliable and that these characteristics of the data are maintained throughout the data life cycle. The data should be collected and maintained in a secure manner, so that they are attributable, legible, contemporaneously recorded, original (or a true copy) and accurate. Assuring data integrity requires appropriate quality and risk management systems, including adherence to sound scientific principles and good documentation practices.”
From MHRA GXP Data Integrity Guidance and Definitions; Revision 1.
Data integrity is a central issue in all laboratories which are regulated by Good Manufacturing Practice (GMP). However, even research laboratories and industries that are not regulated can recognize that the benefits of establishing good data management practices outweigh the costs. The regulatory background will be discussed in this white paper followed by system architecture, which is important in computerized systems such as thermal analyzers.
For all systems which store, process, and retrieve data – such as thermal analyzers – data integrity is paramount. STARe thermal analysis software provides password access-control to the application, assigns user-rights for each user-level, ensures file integrity with electronic records stored in a secure database, and properly logs the audit trail and electronic signatures. STARe software also permits electronic transfer of balance data (STAReX™ connectivity) and automated backups for enhanced data security and efficiency.
Data integrity controls in STARe are available in two options to suit the needs of regulated and non-regulated industries alike:
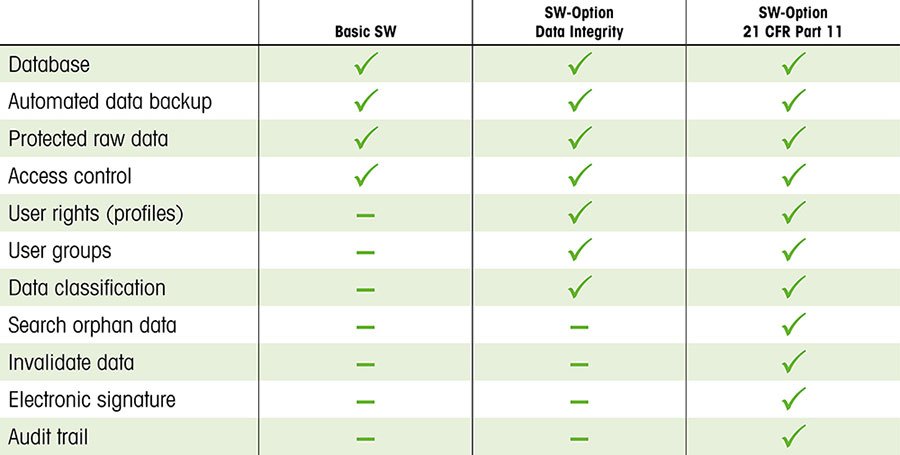 |










