 |
Table of Contents:
TA Tip
- Thermogravimetry and gas analysis, Part 5: TGA-Micro GC/MS
News
- STARe SW V16.20 software
- Photo DSC brings new light into thermal analysis
Applications
- Reproducibility of the enthalpy of melting of an organic powder
- Fast Scanning DSC: Selected application examples
- Determination of caloric effects by TGA/DSC
- Influence of coalescing agents on the curing of polymer dispersions
Reproducibility of the enthalpy of melting of an organic powder
Indium is the material most often used for calibration and adjustment in DSC. METTLER TOLEDO specifies a value of better than 1% for the precision of the enthalpy of fusion of indium (reproducibility for new samples) determined from DSC measurements [1]. Pharmaceutical products, however, differ in many respects in comparison with indium (for example in morphology, thermal conductivity, stability, etc.).
Introduction
In some cases, for example in the pharmaceutical field, it seems desirable to use organic reference materials in order to achieve a better comparison of the reference materials used for calibration and adjustment with the analytical samples. The question then arises concerning the precision with which the enthalpy of fusion of such materials can be determined by DSC.
In this article, we discuss what needs to be taken into account using benzamide as an example. The results show that the precision specified for indium for the enthalpy of fusion determined by DSC (<1%) can also be achieved for organic materials if enough attention is paid to certain points. The precision with which the enthalpy of fusion can be determined by DSC depends on many factors.
These include:
- the state of the DSC (lid, sensor)
- the adjustment of the sample robot
- sample preparation (grinding, compacting, special measures with hygroscopic samples or with samples that contain volatile constituents)
- the thermal contact between the sample and the bottom of the crucible
- the type of crucible (open or closed crucible) and
- the method (heating rate, start temperature, sampling rate).
Some of these points will be discussed in the following sections. Using benzamide as an example, we show that a reproducibility of better than 1% can also be achieved with organic samples.
[…]
References
[1] M. Schubnell, METTLER TOLEDO Collected Applications Handbook: Validation in Thermal Analysis, 110.
Fast Scanning DSC: Selected application examples
The Flash DSC 2+ offers exciting new application possibilities. These are illustrated with the aid of several interesting examples. Besides polymers and organic substances, the instrument can now measure metals and other inorganic materials such as silicate glasses.
Introduction
The Flash DSC 2+ is a further development of the Flash DSC 1, an instrument which can be used for measurements at heating and cooling rates of up to several thousand Kelvin per second. Such high scanning rates are achieved through the use of thin film sensors. The Flash DSC 2+ can be operated with two different sensors. Der UFS 1 sensor is already wellknown from the Flash DSC 1 and can be used up to a temperature of about 520 °C. The UFH 1 sensor can be used up to about 1000 °C and offers higher cooling rates [1]. This article discusses several different applications of the Flash DSC to materials such as metal alloys, silicate glasses and polymers.
[…]
References
[1] J. Schawe, The revolutionary Flash DSC 1: maximum performance for metastable materials, UserCom 32, 12-16.
Determination of caloric effects by TGA/DSC
Besides determining the mass loss, simultaneous thermal analysis using the TGA/DSC enables you to determine caloric effects such as enthalpies of fusion, melting points, and glass transition temperatures using one single instrument. In this article, we discuss two application examples that show the extent to which the DSC signal of a TGA/DSC instrument can provide informative results. The results are compared with results obtained using a DSC instrument.
Introduction
It is often a good idea to use TGA/DSC to investigate samples that exhibit distinct caloric effects above room temperature. In this study, we have looked at two application examples. In the first example, we examined an epoxy resin by TGA/ DSC and then by DSC for comparison in order to determine the glass transition temperature.
The second example had to do with the analysis of the melting behavior of a wax sample that melted between 30 and 70 °C.
[…]
Influence of coalescing agents on the curing of polymer dispersions
Paints and coatings, for example acrylic paints, are dispersions of polymer particles (e.g. acrylic particles) in a solvent (e.g. water). The paints also include numerous different additives which adjust and enhance specific properties such as color, gloss, UV stability, flow behavior, etc. depending on the exact requirements. Coalescing agents play an important role in that they allow polymer dispersions to form perfect films. This article shows how the addition of a coalescing agent affects the film formation of an acrylic dispersion.
Introduction
Paints and coatings consist mainly of pigments, a binder and a solvent. In addition, numerous additives are used to adjust and optimize specific properties such as drying time, flow behavior, film formation, UV stability, gloss, etc. Nowadays, water-based polymer dispersions are widely used as binder/solvent systems and acrylates are often used as polymers (e.g. for acrylic coatings). Film formation with water-based polymer dispersions occurs in three steps (see Figure 1).
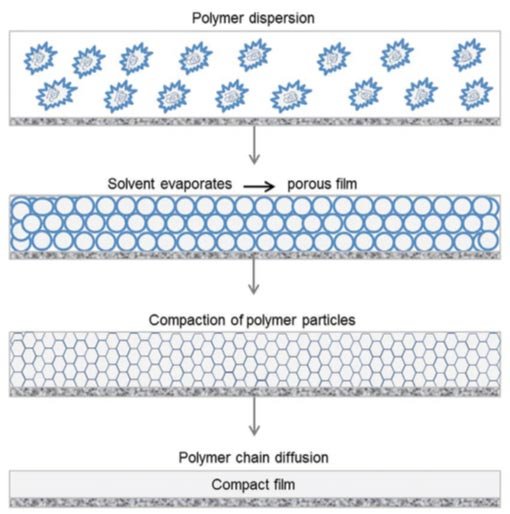 |
Figure 1. Film formation of a polymer dispersion. For details, see text. |
After a water-based polymer dispersion has been applied to a substrate as a thin film, the water first evaporates. As a result, the polymer particles move closer and closer together. At a certain water content, the dispersion collapses and the polymer particles come into contact with each other and form a continuous porous film.
The driving force for the collapse of the polymer dispersion is the lower surface tension of the porous film compared with the surface tension of the polymer particles dispersed in the water. The volumetric polymer content in this stadium is typically about 70%. Evaporation of water continues after the polymer dispersion has collapsed. At the same time, capillary forces act which lead to deformation of the polymer particles and hence to compaction of the polymer film. After compaction, the polymer content of the film is about 90%.
In the final step, the remainder of the water evaporates. At the same time, the boundary layers between the polymer particles are weakened. This enables polymer chains to diffuse from one polymer particle into neighboring particles (interdiffusion).
Once this step has occurred, a compact film is then able to form on the substrate. Details of the processes occurring during film formation can be found for example in [1] and more general information in [2, 3, 4, 5]. Complete film formation can only occur above a so-called minimum film-formation temperature (MFFT).
Below this temperature, the polymer particles are so hard that they are not sufficiently deformed during the drying process. Interdiffusion of polymer chains is then hindered or no longer possible. This leads to the formation of unstable films that are susceptible to cracking or in the worst case to a poorly adhering powdery layer on the substrate.
Polymer dispersions used in paints and coatings have a typical MFFT of about 15 °C. The MFFT corresponds within a few degrees to the glass transition temperature (Tg) of the polymer particles used. Dispersions with MFFTs of about 15 °C form a compact film after drying at 20 °C for example. Since the MFFT corresponds roughly to the glass transition temperature, the paint becomes increasingly sticky or tacky above 20 °C, which is of course undesirable.
Coalescing agents are therefore added to polymer dispersions in order to obtain paints that exhibit a low MFFT (e.g. 15 °C, which is a practical requirement), but that do not become sticky at higher temperatures.
They act as temporary plasticizers for the polymer particles (the Tg and thus the MFFT are lowered). The plasticizers then slowly evaporate from the film after it has been formed. The glass transition temperature of the film gradually increases to the original glass transition temperature of the polymer particles used.
The requirements of a coalescing agent for a polymer dispersion are thus:
- It must act as a plasticizer for the polymer used in the polymer dispersion.
- It must not dissolve in the solvent used for the polymer dispersion (e.g. water).
- It must evaporate much more slowly than the solvent used.
Once there is a systematic (but not exactly known) relationship between the MFFT and Tg, the effectiveness of the coalescing agent on the polymer dispersion can be experimentally investigated. This is usually done using the procedure described in the ASTM Standard D 2354. Alternatively, the influence of a coalescing agent on the Tg can be quickly determined by DSC measurements. In this article, we illustrate the procedure using an acrylic dispersion as an example.
[…]
References
[1] Thilo Jahr, Untersuchung der Filmbildung aus Polymerdispersionen mit Hilfe der forcierten Rayleigh- Streuung, PhD-Thesis, Johannes Gutenberg Universität Mainz, 2002. http://archimed.uni-mainz.de/ pub/2002/0067/diss.pdf
[2] Calculation of Tg and MFFT depression due to added coalescing agents, Progress in Organic Coatings 30 (1997) 179-184.
[3] Minimum Film Forming Temperature, European Coatings Journal, Issue 03/2003, Page 112.
[4] An overview of polymer latex film formation and properties, Advances in Colloid and Interface Science, 86, (2000), 195–267.
[5] Patent EP2247566A2 Efficient coalescing agents.







