 |
Table of Contents:
TA Tip
- Curve interpretation Part 5: TMA curves
News
- New handbooks on thermal analysis
- The new HC103 Moisture Analyzer
- FastTrack™ UV/VIS spectroscopy Speed up your measurements
Applications
- Quality control of olive oil by UV/VIS spectroscopy and DSC
- Influence of water on the glass transition temperature of
- Databases as an aid for the interpretation of DSC curves
- Investigation and identification of constituents of a rubber compound
Quality control of olive oil by UV/VIS spectroscopy and DSC
Olive oil is associated with healthy food due to its high content of Vitamin E, antioxidants, and mono-unsaturated fatty acids. Olive oils are available in different qualities such as extra virgin, virgin, olive oil, and others. In this article, we compare two olive oils of different quality by UV/VIS spectroscopy and DSC.
Introduction
Mediterranean cooking is difficult to imagine without olive oil. Because of its high content of mono-unsaturated fatty acids, vitamin E and antioxidants, olive oil is looked on as a “healthy” oil. Cold- pressed extra virgin olive oil is the highest quality. Olive oils that do not have a precise quality declaration are usually blends of refined olive oil and extra virgin or virgin olive oil.
Olive oils of lower quality contain conjugated dienes and trienes (see Figure 1). These and other compounds are formed as a result of oxidative degradation processes in the oil. The conjugated carbon- carbon double bonds of the dienes and trienes absorb UV light in the wavelength range 200 to 300 nm.
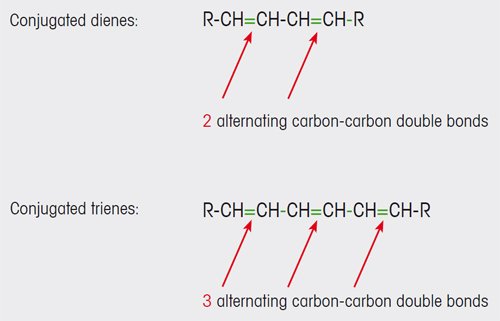 |
Figure 1. Conjugated carbon-carbon double bonds are formed in olive oil as a result of oxidation processes. |
In contrast, substances with non-conjugated double bonds that are also present in extra virgin oil (e.g. unsaturated fatty acids) do not absorb light in this spectral range.
This provides the basis for a simple method to check the quality of olive oil:
- low absorption in the spectral range 200 to 300 nm points to high quality extra virgin oil, and
- high absorption to an olive oil of lower quality.
The International Olive Council has defined three criteria that must be fulfilled for an olive oil to be given the extra virgin label when using UV/VIS spectroscopy for the quality control of olive oils.
The criteria are based on the extinction coefficient Kλ at four different wavelengths, λ (232 nm, 266 nm, 270 nm and 274 nm). Specifically, extra virgin olive oils must fulfill the criteria given in Table 1 [1, 2].
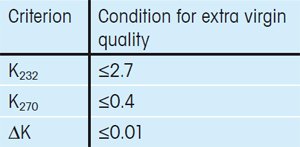 |
Table 1. Spectrophotometric criteria defined by the International Olive Council that must be fulfilled by an extra virgin olive. |
Whereby:
Kλ = Aλ/(c·L) (1)
and
ΔK = K232 – ((K266 + K274)/2) (2)
where Aλ is the absorbance at the wavelength λ, c is the concentration of the sample in the solvent, and L is the path length of the cuvette.
According to the standard described here, the olive oil must be diluted to a one percent solution in cyclohexane (c = 0.01).
[…]
References
[1] EEC Regulation 2568/91 Annex IX (http://eur-lex.europa.eu/).
[2] International Olive Council, IOC COI/T20/Doc.no.19/Rev.3 2010 (www.internationaloliveoil.org).
Influence of water on the glass transition temperature of honey
Honey is well-liked as a spread on bread and as a sweetening agent. The ease of spreading of creamed, noncrystalline or slightly crystalline honey depends on the glass transition temperature. The glass transition temperature itself depends on the water content of the honey. In this article we investigate this relationship using a Swiss floral honey.
Introduction
Honey is a natural product that mainly consists of different types of sugar (fructose 27 to 44% fructose, 22 to 41% glucose) and water (15 to 20%). In addition there are small amounts of pollen, proteins, amino acids, vitamins as well as colorants and aromas [1, 2]. The exact composition is determined by the flowering plants from which the bees collect the nectar.
Sugar can bind large amounts of water through hydrogen bonding. The bound water forms a network with the sugar. The ratio of the contents of fructose and glucose in the honey determines its ability to crystallize and hence the consistency of the honey.
In general, honey with a high glucose content (for example rape honey) crystallizes more easily. Crystallized honey is granular and cannot be spread very well. Creamed honey is no longer easy to spread if its temperature is below the glass transition temperature.
The glass transition temperature of honey depends on the water content. This article describes how this relationship was investigated using DSC measurements of a creamed Swiss floral honey.
[…]
References
[1] http://www.bee-info.com/honey/honey-ingredients.html
[2] https://en.wikipedia.org/wiki/Honey
Databases as an aid for the interpretation of DSC curves
Databases for the interpretation of measurement data are nowadays widely employed in analytical chemistry. In thermal analysis, however, this possibility has hardly been used for the interpretation of measurement curves. In the following article, we present a database in which DSC curves, characteristic values, and FTIR spectra of more than 600 commercially available materials are stored. The data describes about 120 different types of polymers.
Introduction
In many analytical techniques, for example infrared spectroscopy or GC/MS, the interpretation of the measured spectra and hence the identification of substances is performed using databases. The interpretation of DSC curves with the aid of reference curves or characteristic values (such as the glass transition temperature or the melting point and the enthalpy of melting) is much more difficult because both the shape of a measured DSC curve and the characteristic values evaluated depend on the heating rate, the sample mass and the thermal history of the sample. This makes the use of databases for the identification of unknown polymers difficult.
Despite this, databases in which characteristic values and DSC reference curves are stored can be of help for the interpretation of DSC measurements. In the last few years, we have developed a database of this type at the Polymer Institute in Lüdenscheid.
Besides characteristic values, the database also includes DSC curves of different types of polymers measured under standard conditions; the corresponding IR spectra are also stored for each material.
The following sections describe examples showing the identification of polymers by means of DSC curves, characteristic values and IR spectra.
[…]
Investigation and identification of constituents of a rubber compound
The composition and glass transition temperatures of a rubber compound were investigated by TGA and DSC. The tests revealed that the sample was a blend although it was labeled pure NBR (nitrile-butadiene rubber). This article describes the measurements performed to identify the different constituents of the elastomer.
Introduction
The thermal properties, polymer content and glass transition temperature of a rubber compound assumed to consist only of NBR were determined by TGA and DSC. DSC analysis however revealed two glass transitions, which was also confirmed by DMA. The result shows that the material was actually a blend of two different elastomers.
A TGA instrument is often coupled to an infrared spectrometer (FTIR) or a mass spectrometer (MS) in order to understand particular decomposition reactions better and to identify the different gases produced in decomposition reactions during the TGA analysis. In some cases it is difficult to precisely identify the gaseous decomposition products using MS or FTIR because they originate from the decomposition of complex polymers or bitumen. Often, it is almost impossible to identify gases that are simultaneously evolved.
In this particular example, a TGA was interfaced to a GC/MS system (a gas chromatograph coupled to a mass spectrometer) in order to positively identify the main constituents of the rubber compound. Other possible reasons for coupling a TGA to a GC/MS system could be for the analysis of product defects, the evaluation of replacement parts from a different supplier, or the investigation of possible patent infringements with a new product.
To obtain the maximum amount of information about the composition of the gases released during the TGA analysis, an IST16 storage interface was installed between the TGA and the GC/MS system. The IST16 interface allows 16 different fractions of evolved gases to be collected and stored at user-defined times or furnace temperatures during a TGA analysis.
The gas fractions are then injected into the gas chromatograph. Using this arrangement, the decomposition products are first separated on a GC column and then identified in the mass spectrometer and, if desired, quantified. The TGA- IST16-GC/MS combination is shown in Figure 1.
 |
Figure 1. The TGA-IST16-GC/ MS system. |
[…]




