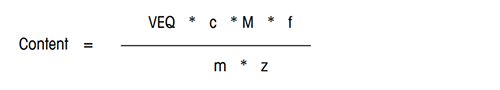|
We created this useful tool so that you can enjoy effortless, reliable, and quick titration calculations. Calculate the mass molarity of solid samples, the concentration of acid and base solutions, the concentration of diluted solutions, titrant consumption, and sample content after a direct titration.
How to Calculate Titration?
The content of the analyte in the sample is calculated according to the following formula:
VEQ = Consumption of titrant [mL]
c = Equivalent concentration of titrant [mol/mL]
M = Molar mass of analyte [g/mol]
m = Sample size [g] or [mL]
z = Equivalent number of the analyte
f = Factor for calculation in the desired unit, e.g. g/L or %