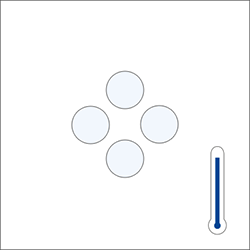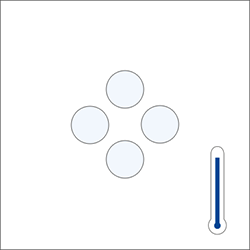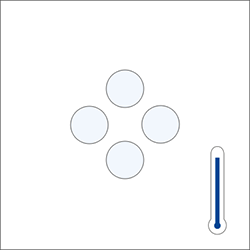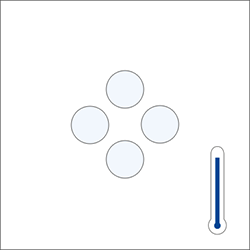
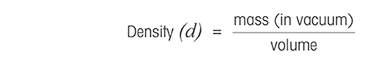Benchtop digital density meters use the same technology as portable digital density meters, the oscillation of a U-shaped glass tube (U-tube). In addition, they feature a built-in Peltier temperature control, which brings the sample to the selected temperature (e.g., 20°C). The temperature control can range from 0 °C to 95 °C. These density meters can reach an accuracy of 0.000005 g/m3 for density.
Some benchtop digital density meters can be connected to sample automation solutions for single or multiple samples, which offer automated sampling, rinsing, and drying. These density meters can often be upgraded into a dedicated automated multi-parameter system combining density, refractive index, pH, color, conductivity, and more to save time, increase data quality, and prevent any alteration of samples between individual analyses.
One of the benefits of digital density meters using the U-shaped glass tube is the small volume of sample required (typically 1.5 mL), which allows for a faster temperature equilibrium of the sample.