Characterization of Delivery Systems by Thermogravimetry
Introduction
A “good” perfume is of course expected to provide a pleasant and distinctive odor. At the same time, the fragrances should remain perceptible for as long as possible at a constant level of intensity.
For this reason, the fragrances in perfumes are now being encapsulated in so-called delivery systems. The release of fragrances then occurs under control, allowing the perception of the perfume to be optimized with respect to intensity and lasting effect.
The encapsulation of fragrances in suitable delivery systems is therefore a topic of great importance for producers of perfumes.
The encapsulation of fragrances in suitable delivery systems is therefore a topic of great importance for producers of perfumes. To identify the most suitable delivery system from the very large number of possible carriers available requires a rapid analytical screening technique that can describe the stability and release performance of a fragrance from the delivery system. Thermogravimetry (TGA) is an excellent technique for this purpose. In this article, the release of Romascone® from three different delivery systems has been investigated using thermogravimetry. Romascone® is a fragrance that finds application in women’s perfumes. The delivery systems utilized three different types of polymeric nanoparticles based on crosslinked vinyl acetate.
Experimental Details
The investigations described here were performed with a METTLER TOLEDO TGA851/SDTAe equipped with the small furnace. Samples masses of typically 8 mg (fragrance and nanoparticles together) were measured in aluminum crucibles. The mass fraction of the nanoparticles made up 40% of the total mass. The purge gas was nitrogen at 20 mL/min. The measurements were performed isothermally at different temperatures.
Theoretical Principles
Evaporation of pure liquids
If volatile compounds (such as fragrances) are measured in the TGA, a continual loss of mass is expected because the furnace is open and an equilibrium state is never reached.
This is because there is a steady transfer of molecules from the liquid phase to the gas phase. Molecules that reach the boundary layer between the liquid and the gas phase are swept out of the TGA furnace by the purge gas. Under isothermal conditions, this results in a constant rate of loss of mass which is determined by the vapor pressure of the compound and the mass transfer at the boundary layer, i.e.
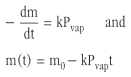
where m is the mass, k is a constant that describes the mass transfer at the boundary layer between the liquid and the gas phase and Pvap is the vapor pressure.
Evaporation of a compound from a binary mixture
In a mixture of two compounds, the chemical potentials of the two compounds in the mixture are reduced compared to the chemical potentials of the individual pure compounds.
In a binary mixture of two ideal noninteracting compounds with molecules of equal size, Raoult’s law predicts that the partial pressure of each compound is proportional to the mole fraction of each species in the mixture, that is:

Here P1 and P2 are the partial pressures of the two compounds, x1 and x2 are their mole fractions and P10 and P20 are the vapor pressures under normal conditions. For real compounds and assuming that only one substance is volatile, the Flory approximation applies and the partial pressure of the volatile component is given by
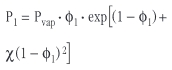
Here Φ1 stands for the volume fraction of the volatile component (solvent) and c for the so-called Flory interaction parameter. For mixing of the two components to occur spontaneously, the mixing enthalpy (expressed here by the Flory interaction parameter, χ) must be small. Typically χ varies between 0 (for good solvents, athermic mixing) and 0.5 (for bad solvents, endothermic mixing). If the interaction parameter is greater than 0.5, demixing of the system is expected. If the density of the two compounds in the mixture is about the same, the volume fraction of the solvent (Φ1) equals its mass fraction (ω1). The rate of mass loss is then given by the equation:

Conclusions
The evaporation of volatile substances from delivery systems can be investigated by thermogravimetry. In the example, the evaporation of Romascone® from nanoparticles based on crosslinked vinyl acetate was investigated.
If the delivery system is in the rubbery-elastic state, the evaporation of Romascone® can be described by the Flory theory (evaporation is limited by the volatility of the volatile substance). If the delivery system is in the glassy state, the evaporation is limited by the diffusion of the volatile substance within the nanoparticles and takes correspondingly longer. The method described here is simple and allows delivery systems to be quickly characterized and optimized.
Characterization of Delivery Systems by Thermogravimetry | Thermal Analysis Application No. UC 255 | Application published in METTLER TOLEDO Thermal Analysis UserCom 25