Influence of the Heating Rate on Decomposition, Metolazone
Sample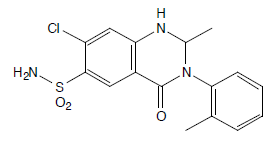
Metolazone
Application
Active ingredient (diuretic)
Conditions
Measuring cells: DSC820
Pan: Aluminum 40 µl, hermetically sealed
Sample preparation: As received, no preparation
DSC measurement: 1. Heating from 30 °C to 300 °C at 20 K/min, 2. Heating from 30 ° to 300 °C at 5 K/min
Atmosphere: Nitrogen, 50 cm3/min
Interpretation
In this example the DSC curves of α-Metolazone measured at two different heating rates are compared. At 20 K/min the melting of the α modification at approx. 227 °C followed by recrystallization in the γ modification can be observed. This melts with accompanying decomposition at approx. 255 °C.
With a heating rate 5 K/min, a much smaller endothermic effect and a depression of the melting point by approx. 7 °C is observed. At 220 °C the hermetically sealed pan bursts (shown as a displacement of the base line).
Evaluation
Only the endothermic peaks are evaluated. To integrate the peaks the baseline types ‘horizontal left’ or ‘horizontal right’ are selected.
| Heating rate | Onset 1, °C | ΔH, J/g | Onset 2, °C | ΔH, J/g |
|---|---|---|---|---|
| 5 K/min | 218.9 | 53.6 | - | - |
| 20 K/min | 227.0 | 83.1 | 255.1 | 31.0 |
Conclusion
Substances which decompose at the melting point can only be characterized to a limited extent by DSC. In such cases, a more reproducible characterization is often possible by using faster heating rates, since the decomposition reaction is moved to higher temperatures. TGA can also be used in the same way to gain additional information, especially when volatile decomposition products are formed.
Influence of the Heating Rate on Decomposition, Metolazone | Thermal Analysis Application No. HB813 | Application published in METTLER TOLEDO TA Application Handbook Pharmaceuticals