Identification Based on Melting Behavior, Polyethylene Glycol
Sample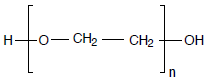
Polyethylene glycols, PEG 400, 1000, 2000, 4000, 6000, 10000, different manufacturers
Application
Inactive ingredient (basic material for ointments and suppositories, solubilizers etc.)
Conditions
Measuring cell: DSC821e with IntraCooler
Pan: Aluminum 40 µl, hermetically sealed
Sample preparation: As received, no preparation
DSC measurement: Held isothermally for 5 minutes at -60 °C, then heated to 160 °C at 10 K/min
Atmosphere: Air, stationary environment, no flow rate
Interpretation
Interpretation Polyethylene glycols are named after their average molecular mass. The melting point increases with increasing chain length, i.e. with increasing molecular mass.
The longer the chain length, the less the melting points of the polyethylene glycols differ from each other. This makes it increasingly difficult to distinguish clearly between samples of high molecular mass.
The melting peak becomes sharper the greater the degree of purity of the substance or, in the case of macromolecules, the greater the degree of uniformity of the size of the crystallites. The differences in the heats of fusion as a function of chain length are relatively small and are also dependent on crystallinity, so that this is not a suitable criterion for differentiation purposes.
Evaluation
Determination of the onset temperatures and the heat of fusion
| Sample | Expected melting range, °C (manufacturer's data) | Onset, °C | ΔH, J/g |
| PEG 400 | -3 to 8 | -14.3 | 156.2 |
| PEG 1000 | 30 to 40 | 37.5 | 194.4 |
| PEG 2000 | 45 to 50 | 49.1 | 214.5 |
| PEG 4000 | 50 to 58 | 54.8 | 207.8 |
| PEG 6000 | 56 to 63 | 56.8 | 212.6 |
| PEG 10000 | - | 59.4 | 218.6 |
Conclusion
Polyethylene glycols can be characterized by DSC by measuring their melting range, even though the differences become smaller with increasing chain length.
Identification Based on Melting Behavior, Polyethylene Glycol | Thermal Analysis Application No. HB812 | Application published in METTLER TOLEDO TA Application Handbook Pharmaceuticals