
Laboratory ORP Sensor
ORP/Redox Electrodes and Probes for Fast and Reliable Measurements
Oxidation-Reduction Potential (ORP) or redox sensors measure the ability of a solution to act as an oxidizing or reducing agent. The ionic potential information an ORP probe obtains is critical for various industrial applications, such as checking drinking water purity, monitoring the anaerobic activity of wastewater, and ensuring consistency in food production processes, such as baking. METTLER TOLEDO manufactures a versatile portfolio of high-quality ORP electrodes for lab and field applications. ORP sensors with silver, platinum, or gold rings offer the possibility to measure redox potentials in media with various chemical properties.
Advantages of METTLER TOLEDO's Laboratory ORP Sensors

The Right Sensor for Your Needs
Whether the ORP sensor is used in the lab or taken to tough outdoor or production environments, METTLER TOLEDO offers a suitable electrode. This means a sensor with the right combination of reference system, junction, metal, and shape to support accuracy in any application, from basic day-to-day monitoring to highly specialized workflows.

Built to Last
METTLER TOLEDO ORP sensors not only guarantee high performance, but a correct pairing of materials and technologies increases their durability and extends their lifetime ̶ provided the correct sensor for each lab or field application is used. Our shaft materials warrant for the robustness of the lab ORP sensors even in harsh or near-production environments.
Adapts to Your Needs
Laboratory ORP electrodes with different lengths support easy and highly accurate redox measurements of any sample size.

Platinum Ring Sensors
Inert platinum ring-based redox sensors are designed to cover most applications, while an ARGENTHAL™ reference system provides steady reference potential and contamination-free junctions.

Silver Ring Sensors
Laboratory ORP/redox sensors with a silver sensing ring provide accuracy in argentometric applications.
Gold Ring Sensors
ORP/redox sensors with a gold sensing ring are best for highly oxidizing samples.
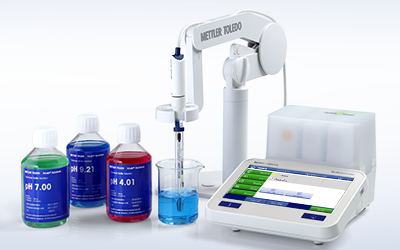
All-In-One Solution
METTLER TOLEDO provides complete electrochemistry systems, from meters and sensors, to calibration and verification standards, and software. Create an effortless system with seamless measurement, data transfer, and automation.
Explore Our Services - Tailored to Fit Your Equipment
We support and service your measurement equipment through its entire life-cycle, from installation to preventive maintenance and calibration to equipment repair.
Support & Repair

FAQs
What is a laboratory ORP sensor?
Oxidation-Reduction Potential (ORP) or Redox potential sensors are used to monitor chemical reactions, to quantify ion activity, or to determine the oxidizing or reducing properties of a solution. ORP is a measurement of the electrical potential of a redox reaction, determining the amount of oxidation or reduction that takes place under existing conditions. METTLER TOLEDO provides reliable ORP sensors for applications in the laboratory and the field.
How does a lab ORP electrode work?
An ORP measurement setup consists of an ORP electrode and a reference electrode, in much the same manner as a pH measurement.
The principle behind the ORP measurement is the use of an inert metal electrode (platinum, sometimes gold or silver), which, due to its low resistance, will give up electrons to an oxidant or accept electrons from a reductant. The ORP electrode will continue to accept or give up electrons until it develops a potential, due to the build-up charge, which is equal to the ORP of the solution.
ORP electrodes measure the redox potential according to the Nernst half-cell potential equation:
E = Eo + (2.3RT / nF) x (log [aOx] / [aRed])
Where:
- E = electrode potential measured
- Eo = voltage specific to the system under analysis.
- R = universal gas constant
- T = Absolute Temperature (K)
- n = number of electrons involved in equilibrium between the oxidized & reduced species.
- F = Faraday constant (96500 coulombs)
- [ ] = denotes activity of bracketed ions
What are the advantages of the ARGENTHAL™ reference system for lab ORP sensors?
In order to prevent the stripping of Ag from Ag wire the improved type of reference element, the ARGENTHAL™ reference element was created. The ARGENTHAL™ reference element consists of a small cartridge filled with AgCl particles that provide the silver ions for the chemical reaction at the lead-off wire. This cartridge contains enough AgCl to last the lifetime of the electrode.
How to store laboratory ORP sensors correctly?
After use, rinse the electrode well with distilled water and close the SafeLock™. ORP electrodes should be stored in the wetting cap filled with a reference electrolyte (often 3 mol/L KCl) or InLab storage solution. Store the half-cell dry. The electrode should be stored upright and at room temperature.
Check user manuals for the necessary information about the storage of the ORP sensors.
How to clean a lab ORP sensor’s junction?
Several factors can lead to the blockage of ORP sensor’s diaphragms. In particular, junctions made of ceramic, or other porous material are prone to clogging. The most frequent reasons are listed here together with the respective cleaning procedures:
Blockage with silver sulfide (Ag2S): if the reference electrolyte contains silver ions and the sample being measured contains sulfides, the junction will get contaminated with a silver sulfide precipitate. To clear the junction of this contamination, clean it with 8% thiourea in 0.1 mol/L HCl solution for 5-60 minutes (Thiourea Cleaner is available from METTLER TOLEDO).
Blockage with silver chloride (AgCl): the silver ions from the reference electrolyte can also react with samples that contain chloride ions, resulting in an AgCl precipitate. This precipitate can be removed by soaking the electrode in a concentrated ammonia solution (35% NH3 aq.).
Blockage with proteins: junctions contaminated with proteins can often be cleaned by immersing the electrode in a pepsin/HCl (5 % pepsin in 0.1 mol/L HCl) solution for several hours (Pepsin-HCl Cleaner is available from METTLER TOLEDO).
Other junction blockages: if the junction is blocked with other contaminations, try cleaning the ORP sensor in an ultrasonic bath with water or with a 0.1 mol/L HCl solution.
Which sensor model should be used for ORP measurements?
Laboratory redox electrodes with a platinum ring are the "standard" ORP sensors. We have sensors with different geometries and junctions as well (e.g. InLab Redox Micro, InLab Redox Pro). Redox electrodes are only used if anything in the sample undergoes a chemical reaction with platinum -- the idea behind the noble metal ring is to not be involved in any chemical reaction. One example where a platinum redox electrode is not recommended is concentrated hydrochloric acid because Pt-Cl complexes can be produced.
Why is calibration not needed for lab redox sensors?
Measuring redox means to measure the reduction potential of the solution. The raw value (mV reading) is the final result.
If the redox electrode is verified by measuring in 220mV buffer solution and if it is not within 220 ± 20 mV, the sensor must be cleaned (and not calibrated).
What to do if the ORP sensor verification fails?
The expected value for the Redox sensor is 220 ± 20 mV. If this condition is not met, it is suggested to clean the metallic ring or pin, using a wet tissue, followed by rinsing with distilled water and then re-measure mV value in redox buffer 220 mV.
Another way to clean and remove deposits from the metal ring is to condition it with 0.1 mol/L HCI. Also in some cases, a change of reference electrolyte is recommended.
Can a lab pH probe be used for redox measurements?
A pH probe cannot be used for redox measurements. The working principles behind the sensors (pH and Redox) are different.
ORP is a measurement of the electrical potential of a redox reaction, and how much oxidation or reduction takes place under existing conditions. The ORP measurement can be made using the millivolt mode of a pH meter. The sensing element is metal here, typically platinum.
pH value is the measurement of the activity of the hydrogen ions (protons) or hydroxyl ions in an aqueous solution. The sensing element here is glass sensitive membrane. The quantitative difference between acidic and alkaline substances can be determined by performing pH value measurements.
Hence, a pH probe cannot be used for redox measurements. This can be explained by a good example below.
Our redox standard 220 mV has a pH 7. If you measure in ORP mode (mV mode) with a platinum ring sensor, you get around 220 mV. But if you measure with a pH electrode, the meter displays around 0 mV. The reason is that the two different sensors are sensitive to different species in the solution: the redox electrode to metal ions, and the pH electrode to protons.
When are relative mV measurements done?
It might be that someone wants to correct the reading for any offset, for example, to know the potential against a hydrogen standard electrode instead of the Ag/AgCl reference. Therefore, relative mV measurements are performed, and one needs to enter the offset in the measurement parameters.
What are the common ORP applications?
One of the biggest applications that use ORP includes water disinfection. Municipal drinking water supplies, for example, use strong oxidizers such as chlorine to kill bacteria and other microbes and to prevent their growth in water supply lines.
ORP measurement can be found in diverse applications such as disinfection, wine-making, electroplating, and mining. Redox reactions are common practice in industrial wastewater treatment for either the reduction or oxidation of components before discharge. Cyanide wastewater treatment is a common example of an oxidation reaction in metal processing applications.
Chromate is a commonly used chemical in the electroplating of metals to alter chemical properties. The compound is toxic and needs to be removed from wastewater to limit environmental release. Chromate reduction from hexavalent chromium to trivalent chromium is pH controlled at acidic conditions and monitored with ORP.
Are there METTLER TOLEDO Redox sensors for special volume applications?
Yes, for lab applications that involve long vessels for ORP measurements, we have InLab Redox-L. The extra-long shaft allows measurements in deep vessels, barrels or pilot reactors. For samples available in small volumes then InLab Redox Micro is the choice. The slim shaft diameter allows measurements of very small sample volumes.








