Simple Determination of the Thermal Conductivity of Polymers by DSC
Simple DSC measurements can be used to rapidly determine the thermal conductivity of polymers and other materials with similarly low values with an accuracy of about ±10 to ±20%.
A procedure for doing this was published in 1985 by Hakvoort and van Rejien. In this method, the melting behavior of a pure metal on top of a cylindrical sample or disk is measured.
To simplify sample handling, we have modified the method by containing the metal in a crucible. The thermal conductivities of 11 polymers were measured and the results compared with literature values. The values we obtained were on average about 4% lower than literature values. This deviation is small if you take into account the general measurement uncertainties associated with thermal conductivity measurements.
Simple DSC measurements can be used to rapidly determine the thermal conductivity of polymers and other materials with similarly low values with an accuracy of about ±10 to ±20%.
A procedure for doing this was published in 1985 by Hakvoort and van Rejien. In this method, the melting behavior of a pure metal on top of a cylindrical sample or disk is measured.
To simplify sample handling, we have modified the method by containing the metal in a crucible. The thermal conductivities of 11 polymers were measured and the results compared with literature values. The values we obtained were on average about 4% lower than literature values. This deviation is small if you take into account the general measurement uncertainties associated with thermal conductivity measurements.
Introduction
Hakvoort and van Reijen [1] proposed an interesting method to determine the thermal conductivity of solid materials. This consisted of putting a pure metal (e.g. indium or gallium) on the upper circular end surface of a sample in the shape of a right circular cylinder or disk, and then placing the disk (without a crucible) directly on the DSC measuring sensor. During heating, the metal reaches its melting point and the temperature remains constant while the metal melts. The temperature of the upper end surface of the disk is thus known at this instant. The temperature of the lower end surface of the disk and the heat flowing into the disk are measured by the DSC. The thermal conductivity of the sample can then be calculated from the temperature difference between upper and lower end surfaces of the disk and the heat flow.
Besides DSC, many instruments are nowadays available that have been specially designed to determine thermal conductivity. The advantage of DSC, however, is that the specific heat capacity can also be measured with the same instrument. This allows the thermal diffusivity (λ/(ρcp) of a material to be determined. The method outlined above using the metals has recently been reintroduced to determine the properties of composite materials [2] and new materials [3].
In the following article we will describe a simple DSC method that allows the thermal conductivity of polymers to be rapidly determined typically with a measurement uncertainty of 10%.
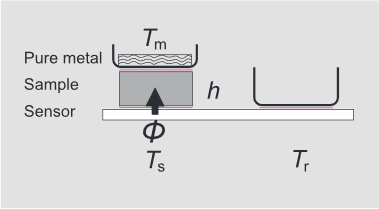
Figure 1. Schematic diagram of the sample arrangement on the DSC sensor. h: is the height of the sample cylinder; φ the heat flow that flows from the sensor into the sample; Tm the temperature of the metal melt, Ts the sensor temperature under the sample; Tr the temperature of the reference sample. The reference crucible is empty. A crucible of the same type containing the pure metal is placed on the sample. The spaces between the crucible-sample and sample-sensor interfaces are filled with heat transfer oil (red lines).
Theoretical Background
Under stationary conditions, the heat flow, φ, through a body with a thermal resistance, Rs, is proportional to the temperature difference, ΔT:
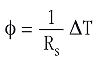
The thermal resistance, Rs, of the material is given by the material-dependent thermal conductivity and the geometry of the body:
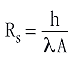
Here λ is the thermal conductivity, A the cross-sectional area, and h the length of the body.
With cylindrical samples of diameter, D, and height, h,
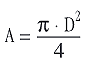
Figure 1 shows the setup used to determine the thermal conductivity of a material by DSC. The heat flow from the sensor to the pure metal depends not only on the thermal resistance of the sample, but also on the thermal resistances at the sensor-sample (R1) and sample-metal (R2) interfaces. Equation (1) must therefore be rewritten as follows:
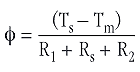
To ensure that the thermal resistances R1 and R2 were reproducible, the spaces at the interfaces were filled with heat transfer oil. It can therefore be assumed that R1 and R2 are independent of the sample if the same sample cross-section is always used.
RT = R1 + R2
Rs and hence the thermal conductivity of the sample can only be determined if φ, RT, Tm and Ts in eqs 4 and 5 are known. The value of Tm during melting is known because a pure metal is used. The values of φ and Ts are obtained from the DSC measurement, and RT can be determined by performing several measurements. If RT is much smaller than Rs, then RT can even be neglected and λ can be determined from a single melting curve. Combining eqs 1 and 2 gives eq 6:
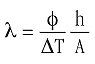
Equation 6 is valid only during melting. ΔT is then the difference between the temperature Ts at a time t and the melting point of the metal (i.e. the temperature of the beginning of melting, Tonset). The corresponding heat flow φ is the difference between the heat flow at the same time t and the heat flow at the beginning of melting (see Figure 2).
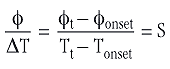
S is therefore the slope of the linear side of the melting peak.
Conclusions
The determination of the thermal conductivity of polymers can easily be performed by DSC; the measurement uncertainty is usually less than 20%. The value of -4% obtained for the deviation of the measured values from the literature values is comparable with the result of the interlaboratory study based on ASTM Standard E1952 (-6% mean measurement deviation with a repeatability, r, of 12%). The standard procedure is based on several temperature-modulated DSC measurements. It requires an accurately known reference substance and the evaluations involve complex mathematical calculations. Despite the significantly greater amount of time and complexity of the ASTM E1952 procedure, the thermal conductivity values measured are not more accurate than those obtained with the simple method used here. Furthermore, only two polymers were measured in the interlaboratory study of the E1952 Standard. Here, in contrast, eleven different materials were measured and a much broader thermal conductivity range was determined.
The sample cylinder or disk should be about 1 to 3 mm in height and must have the same diameter as the bottom of the crucible containing the pure metal. In the molten state, the metal must completely cover the bottom of the crucible. If gallium is used as the reference metal, the aluminum crucible must be coated internally with a lacquer in order to prevent alloy formation. The spaces between the sensor and sample and the sample and crucible are filled with oil so that thermal resistances are as reproducible as possible. Furthermore, the end faces of the sample disks must be flat. The DSC should be adjusted in the usual way with just the crucible and the metal (i.e. without the cylindrical sample) so that the enthalpy of melting and the melting temperature agree with literature values.
In general, we recommend that you perform the determination according to eq 9, using a measurement with and without the sample.
Simple Determination of the Thermal Conductivity of Polymers by DSC | Thermal Analysis Application No. UC 226 | Application published in METTLER TOLEDO Thermal Analysis UserCom 22