Purity Determination, Phenacetin + 4-Aminobenzoic Acid
Sample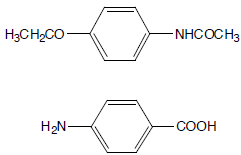
Purity set NBS:
Phenacetin + 0.7 mole % 4-Aminobenzoic acid
Phenacetin + 2.0 mole % 4-Aminobenzoic acid
Phenacetin + 5.0 mole % 4-Aminobenzoic acid
Application
As a standard for purity determination
Conditions
Measuring cell: DSC820
Pan: Aluminum 40 µl, hermetically sealed
Sample preparation: As received, no preparation
DSC measurement: Heating from 100 °C to 160 °C at 10 or 5 K/min, Heating from 110 °C to 150 °C at 2.5 or 1.25 K/min
Atmosphere: Nitrogen, 50 cm3/min
Interpretation
The diagram shows the melting curves of the pure sample and the contaminated samples of phenacetin. The melting peak becomes broader and shifts to lower temperature with increasing impurity. In addition, the eutectic peak at about 114 °C becomes increasingly noticeable. The purity determination is based on the van't Hoff equation, which states that the melting point depression is proportional to the mole fraction impurity (see the relevant literature). The diagram showing the equilibrium melting temperature from the melting curve plotted against the reciprocal of the fraction melted (F) is known as the 1/F plot. After allowing for the heat of fusion of the eutectic (linearization correction), a linear relationship is generated from which the purity can be determined.
Evaluation
The purity of each of the samples given below was determined at the heating rates indicated, according to the method of van't Hoff. The melting curve was evaluated in the range from 10% to 50% of the peak height.
| Substance | Purity 1, mole % | Heating rate, K/min | |||
|---|---|---|---|---|---|
| 1.25 | 2.5 | 5 | 10 | ||
| Phenacetin pure 2 | 99.9 +/- 0.1 | 99.97 | 99.98 | 99.96 | 99.97 |
| +0.7 mole % PABS | 99.3 +/- 0.2 | 99.39 | 99.29 | 99.34 | 99.50 |
| +2.0 mole % PABS | 98.0 +/- 0.2 | 98.35 | 98.39 | 98.49 | 98.40 |
| +5.0 mole % PABS | 94.9 +/- 0.2 | 98.30 | 97.07 | 96.79 | 97.04 |
1 certified value
2 mean value of 3 measurements
Conclusion
The DSC purity determination gives rapid results. The method may only be applied, however, if certain conditions are fulfilled. In particular, only relatively pure substances (>98%) should be characterized with this technique.
A number of different parameters must be considered when developing a method for purity determination. These include particle size, sample weight, heating rate and choice of the data points to be used for the calculation. A variation in the particle size can cause broader or irregular melting peaks, since the heat flow through larger crystals requires more time. A large sample weight has the same effect because of the increased sample thickness. On the other hand, trapped air in fine powders can lead to a delayed transfer of heat between the individual crystals.
Purity Determination, Phenacetin + 4-Aminobenzoic Acid | Thermal Analysis Application No. HB836 | Application published in METTLER TOLEDO TA Application Handbook Pharmaceuticals