Decomposition of Copper Sulfate Pentahydrate (Tutorial) by TGA-MS and TGA-FTIR
Introduction
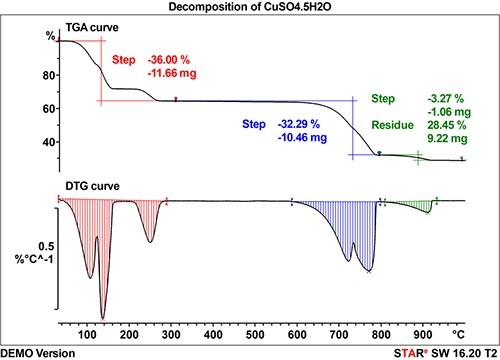
The thermal decomposition of copper sulfate pentahydrate (CuSO4.5H20) is used to demonstrate the advantage of an online coupling technique. CuSO4.5H20 loses its water of crystallization in several steps. The resulting anhydrous copper sulfate decomposes in three further reaction steps as given by the following equations:.
(1) 2 CuSO4 → Cu2(SO4)O + SO3
(2) Cu2(SO4)O → 2 CuO + SO3
(3) 2 Cu0 → Cu20 + ½ 02
Sample: Copper sulfate pentahydrate (CuSO4·5 H2O)
Measuring cells: TGA-MS and TGA-FTIR
 |
Decomposition of Copper Sulfate Pentahydrate (Tutorial) by TGA-MS and TGA-FTIR | Thermal Analysis Handbook No.HB601 | Application published in METTLER TOLEDO TA Application Handbook Evolved Gas Analysis
May 02, 2024
May 30, 2024
Apr 25, 2024
Jun 27, 2024
Oct 31, 2024