 |
This melting point and dropping point guide explains the measurement principle of automated melting & dropping point analysis, and gives tips & hints for better measurements and performance verification.
In order to characterize a material aside from chemical analysis, primarily physical methods allow us to differentiate between, identify and classify substances or to provide descriptions of quality. The thermal characteristics of a substance or mixture of substances, such as melting point and dropping point, provide valuable and accessible information in this respect.
- Learn about Melting Point, Pharmacopeia Melting Point, Thermodynamic Melting Point, Dropping Point and Softening Point
- Get Tips & Hints from our expert for correct sample preparation and measurement
- Learn how to check your instrument with certified reference substances
Melting Point and Dropping Point Guide
1. Introduction
In order to characterize a material aside from chemical analysis, primarily physical methods allow us to differentiate between, identify and classify substances or to provide descriptions of quality. The thermal characteristics of a substance or mixture of substances, such as melting point, provide valuable and accessible information in this respect. The melting point is the temperature at which the solid phase changes to the liquid state. Such accurate data is associated with the equally important, but more empirical, thermal characteristic: dropping or softening point of industrial or naturally-occurring products. These empirical values are dependent on the measurement method used and thus require a sensible standardized procedure. The exact thermal characteristics of almost all pure organic and inorganic substances, however, can be found in comprehensive tables.
For almost 5 decades METTLER TOLEDO has provided instrumental solutions for the automatic determination of the thermal values melting point, dropping and softening point. The melting point and Dropping Point Excellence line, METTLER TOLEDO’s latest release of compact instruments for thermal characterization, support the complete analytical workflow with innovative solutions. These are highlighted in this guideline in addition to fundamental knowledge about melting, dropping and softening point determination and practical application tips for daily use.
2. The Melting Point
- 2.1 General Considerations
Determination of melting point is one of the oldest methods of identification and testing, particularly for organic substances. Melting point is easy to determine and, since it is a numerical property, can be conveniently tabulated and classified. Since melting point is highly dependent on purity, it can also be used for evaluating the quality of substances. Pure crystalline substances change to the liquid state at a precisely defined temperature, which is specific for each crystalline material. Crystalline and molten states of the same material can only exist at the same time at the melting point. Automatic melting point determination methods make use of these phenomena, which become visible during the change in aggregate state.
Pure substances melt at a highly-defined temperature whereas impure, contaminated substances generally exhibit a large melting interval. The temperature at which all material of a contaminated substance is molten is usually lower than that of a pure substance. This behavior is known as melting point depression and can be used to obtain qualitative information about the purity of a substance. - 2.2 Automatic Melting Point Determination: The METTLER TOLEDO MP Excellence Instrument Line
- 2.3 Automatic Melting Point Determination: The Measurement Principle
- 2.4 Pharmacopeia Melting Point
- 2.5 Transmission Intensity Curve
- 2.6 Thermodynamic Melting Point
3. Application Tips & Hints for Accurate Melting Point Determination
- 3.1 Instrument Calibration and Adjustment
If we want to make sure that the melting point instrument is providing the correct results, we need to verify its measurement accuracy. In the previous chapter we learned that we cannot measure the sample temperature directly using a certified thermometer. Therefore, in order to check the temperature accuracy, we use reference substances ideally with certified temperature values. Thus, we can compare nominal values including tolerances with actual measured values. If calibration fails, which means if the measured temperature values do not match the range of the certified nominal values of the respective reference substances, we need to adjust the instrument.
[…] - 3.2 Quick and Reliable Sample Preparation: The Capillary Filling Tool
- 3.3 Melting Point Capillary Sample Preparation
- 3.4 Sample Preparation: Bad Example
- 3.5 Sample Preparation: Good Example
- 3.6 Sample Measurement – Tips & Hints
- 3.7 Melting Point Standards Overview
- 3.8 Melting Point Measurement Results
4. Melting Point Workflow Support by LabX® PC Software
LabX PC software integrates almost the complete laboratory instrument portfolio of METTLER TOLEDO including analytical balances, density meters, refractometers, titrators and melting point instruments MP70 and MP90. Integration with LabX means that each analytical workflow performed on the each instrument is controlled by LabX. Results are securely stored in the LabX database, which can be archived or restored when required. Like the common OneClick® interface of the METTLER TOLEDO instruments, LabX provides a unique interface for all integrated instruments on the PC. Beyond instrument and data management, LabX manages instrument users based on a comprehensive management system complying with all FDA requirements. Measurement tasks can be scheduled and issued to the relevant instrument according to the lab's schedule. The operator has the appropriate user rights to work at the specific instrument. LabX supports efficient workflow execution on various different analytical instruments from METTLER TOLEDO and facilitates data management by centralized storage and full 21CFR part 11 compliant audit trail.
- 4.1 Melting Point Workflow Integration and Support by LabX
- 4.2 Melting Point Screening Supported by LabX
5. Fundamentals of Dropping & Softening Point Determination
- 5.1 General Consideration
Synthetic but also naturally-occurring products, which are important raw materials for various industry segments, do not show a defined melting point. They include ointments, synthetic and natural resins, edible fats, greases, waxes, fatty acid esters, polymers, asphalt and tars. These materials gradually soften as the temperature rises and melt over a relatively large temperature interval. Generally the dropping or softening point test is one of the few easily achievable methods available to thermally characterize such materials. To ensure comparable results, standardized test equipment and conditions, as well as appropriate sample preparation, are required. - 5.2 Automatic Dropping and Softening Point Determination: The METTLER TOLEDO DP Excellence Instrument Line
- 5.3 Automatic Dropping and Softening Point Determination: The Unique Detection Principle
- 5.4 Automated Dropping Point Measurement by Video Based Image Analysis
- 5.5 Automated Softening Point Measurement by Video Based Image Analysis
- 5.6 International Dropping and Softening Point Standards
6. Application Tips & Hints for Accurate Dropping & Softening Point Determination
- 6.1 Instrument Calibration and Adjustment
If we want to make sure that our dropping point instrument functions correctly, we need to verify its measurement accuracy. This procedure is called calibration and in the following section valuable tips and hints are given to help perform the calibration correctly. As with melting point instruments, in dropping point instruments the sample temperature cannot be measured directly by the use of a certified thermometer. This would falsify the measurement as a significant portion of the heat transferred from the furnace to melt the sample would be used instead to heat the thermo element.
[…] - 6.2 Sample Preparation
- 6.2.1 DP Excellence Accessory Box
- 6.2.2 Efficient and Reliable Sample Preparation: The Sample Preparation Tool
- 6.2.3 DP Excellence Sample Holder and Standard Compliant Cups
- 6.2.4 Solid Samples
- 6.2.5 Lubricant Greases
- 6.2.6 Bitumen, Pitch
- 6.2.7 Resins, Rosins
- 6.2.8 Waxes
- 6.2.9 Liquid Samples or Samples that Require Cooling Prior to Measurement
7. Dropping and Softening Point Measurement Results
- 7.1 Dropping Point of Edible Oils and Fats Measured on DP90 Excellence
This table provides an overview of dropping point test results of edible oils & fats measured on a DP90 Excellence instrument from METTLER TOLEDO. The measured dropping point temperatures range from minus 6 °C up to 50 °C. Each test was repeated several times in order to achieve a significant repeatability value that could be used for result assessment.
[…] - 7.2 Dropping Point of Fats and Waxes Measured on DP70 Excellence
- 7.3 Dropping Point of Lubricant Greases
- 7.4 Softening Point of Bitumen
- 7.5 Softening Point of Resins
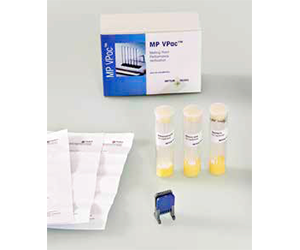
8. Performance Verification with MP VPac™
- 8.1 Ready-to-use Kit of Traceable Reference Substances
Performance verification by temperature calibration is the recommended workflow to ensure faultless routine operation of a melting point instrument and to secure result reliability. The MP VPac provides 3 different certified reference substances in prefilled, sealed capillaries for a simple method of verifying instrument accuracy over the temperature range 40 to 230 °C.
No sample grinding and time-consuming manual upfront filling of melting point capillaries is required. Just take the pre-filled capillaries and insert them into the MP instrument furnace, as in a normal method. The preprogrammed calibration method can be started directly without any modification.
Each of the three reference substances included in the MP VPac comes with a certificate that states the certified temperature value including measurement uncertainty. The purity is routinely checked by DSC measurements. The subsequently measured melting point is therefore a reliable way of checking the temperature accuracy of the instrument and whether an adjustment is required.
- 8.2 Do-it-yourself Service
- 8.3 Reference Substances








