 |
Manual sample processing using flasks can introduce error into lab processes that lead to Out-of-Specification results. A new METTLER TOLEDO article explores 10 reasons to leave error-prone manual workflows behind and create greater accuracy and efficiency in routine sample preparation.
METTLER TOLEDO’s new, free download “10 Reasons to Say Goodbye to Inaccurate Concentrations” describes an alternative to subjective volumetric workflows gravimetric sample preparation. This innovative method enables accurate solution preparation in less time with fewer resources for significant cost savings and productivity enhancement.
Create only what you need
The list of benefits is compelling. It includes being able to create exactly the amount of solution needed rather than being constrained by discrete flask volumes. This helps to conserve often expensive materials and reduces disposal costs. Additionally, an ability to add liquid based on the objective weight of a dosed solid means accurate concentrations every time. Operators spend less time and energy scooping solids to meet tolerances, enhancing process ergonomics.
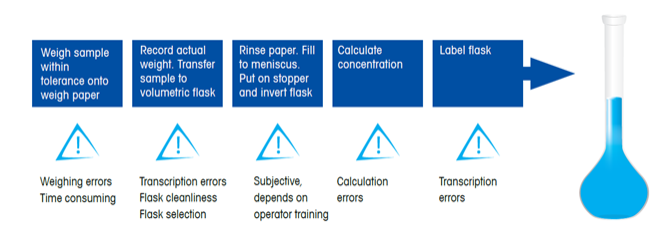 |
Volumetric preparation is highly manual, subjective and variable. |
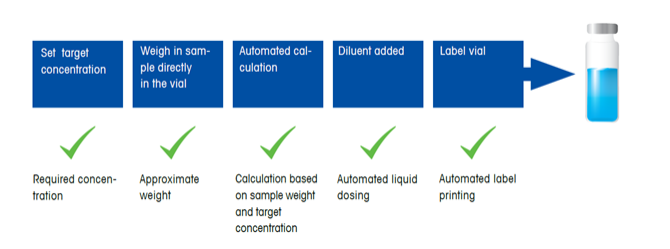 |
Automated dosing eliminates subjectivity and variability. |
Easily convert your balance
"10 Reasons" also describes the ease with which various balance models can be converted to support gravimetric sample preparation. The improvements in data collection, labeling, and traceability that this method offers makes it a truly viable, cost-effective alternative to subjective flask reading.
An accepted method
Fortunately, this innovative method is widely accepted and has been described in the United States Pharmacopeia Chapter <1251>. For more on gravimetric sample preparation and its benefits, download the article here.
 |
Errors in volumetric sample preparation can lead to out-of-specification results. |