Total Decomposition, Malonic Acid
Sample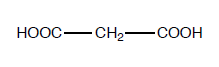
Malonic acid
Application
Inactive ingredient
Conditions
Measuring cells: DSC820 or TGA850
Pan: Aluminum 100 µl, with pierced lid
Sample preparation: As received, no preparation
DSC measurement: Heating from 30 °C to 300 °C at 20 K/min
TGA measurement: Heating from 30 °C to 300 °C at 20 K/min, blank curve corrected
Atmosphere: Nitrogen, DSC: 50 cm3/min, TGA: 80 cm3/min
Interpretation
After melting at about 137 °C, the substance decomposes to acetic acid vapor in an endothermic process with the liberation of CO2. This can be seen in the TGA curve as a 100 per cent weight loss as well as in the DSC curve as an endothermic decomposition peak. The displacement of the baseline is a result of the change of mass and is not caused by a change in the specific heat capacity (cp)
Evaluation
| DSC | Onset, °C | ΔH, J/g |
| Transformation | 103.8 | 20.0 |
| Melting | 137.1 | 222.2 |
| Decomposition | 174.9 | 281.5 |
| TGA | Step, % | Evaluation range, °C |
| Decomposition | 99.99 | 35 to 220 |
Conclusion
Decomposition can be detected with DSC and TGA by a heat change or a loss of weight. Further investigation of the decomposition products requires the use of combination techniques such as TG-MS or TG-FTIR.
Total Decomposition, Malonic Acid | Thermal Analysis Application No. HB846 | Application published in METTLER TOLEDO TA Application Handbook Pharmaceuticals