As a proven end-to-end pharmaceutical track and trace supplier, we provide hardware, software, OEM solutions and integration kits to support serialization and aggregation regulations. Using our many years of experience, we can help implement an effective track and trace program. Our single source project management services take you through the entire process, from pre-sales to installation and ongoing operations support.


This powerful solution combines proven item serialization with high precision weighing using FlashCell™ EMFR technology and visual inspection of the product label and artwork. The compact design features a single HMI for intuitive operation.
System Design
Number of Reading Devices

This software provides unified line control for every sensor, camera, printer, and code reader involved in the aggregation process. Adaptable to multiple aggregation scenarios, it simplifies supply chain management and warehouse prosesses.
System Design
Number of Reading Devices

PLM Direct is the right software choice for serialization and aggregation running on a dedicated single production line. Data exchange with Level 3 and 4 systems is handled using standard XML formats, for easy integration into existing processes.
System Design
Number of Reading Devices

PLM Mark & Verify helps to prevent product recalls and maintains brand reputation by checking presence, reference, and quality of packaging and labeling. Multiple inspection options are available, e.g. code, label, blister, or text inspections.
System Design
Number of Reading Devices

This software offers global control, serving as the unified interface to help monitor and manage serialization. Pre-defined serialization scenarios simplify the production process
System Design
Number of Reading Devices

Site-wide management of production lines, enabling data visibility of the entire Track and Trace process. The PSM further provides advanced connectivity to ERP or MES systems, expanding existing processes for inventory management or line control.
System Design
Number of Reading Devices

This compact system prints and inspects labels using Smart or High-Resolution Camera technology. Faulty labels are detected and can be removed before they are applied to products, ensuring only correct labels are used.
System Design
Number of Reading Devices

Designed to effectively serialize small batches or oversized products by manually sliding the product’s carton along the print unit. A unique code is printed, verified, and serialized by a Smart Camera.
System Design
Number of Reading Devices
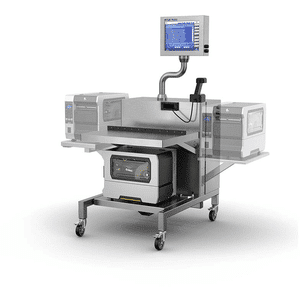
Supports item-by-item manual aggregation of products into bundles, cases or pallets for compliance with Track and Trace labeling. The T15 is portable and therefore an ideal solution for rework on the production floor.
System Design
Number of Reading Devices

Perform semi-automatic aggregation of serialized packages to a case and optionally to a pallet. Verify product codes and label artwork for additional quality control measures using a High-Resolution Camera.
System Design
Number of Reading Devices
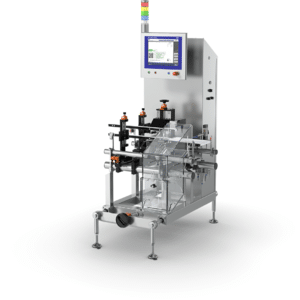
Compact serialization system for easy integration into production lines. Performs all functions for serialization, including the marking, recording, and verification of all serialization data.
System Design
Number of Reading Devices

Marking and verifying print quality on product packaging with three verification types available: Presence, Quality, and Reference Check. Integrates directly on production lines or inside packaging machines, connecting easily with existing printers.
System Design
Number of Reading Devices
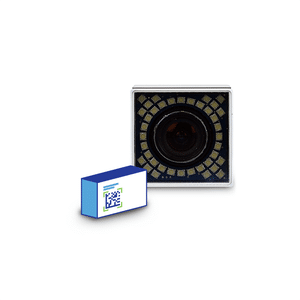
Serialize a wide range of oriented items, such as cartons, bundles, or labels using up to 4 smart cameras. Industry proven, modular and compact components facilitate easy integration into various production lines or packaging machines.
System Design
Number of Reading Devices
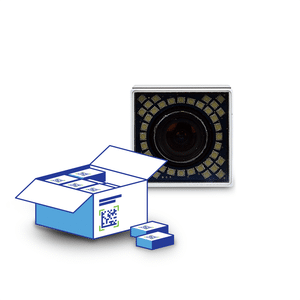
Aggregate a wide range of products item-by-item in cases or pallets using up to 3 Smart Cameras. Industry proven, modular and compact components facilitate easy integration into various production lines or packaging machines.
System Design
Number of Reading Devices

Installs into existing or new case packers or palletizers for improved process efficiency allowing for the aggregation of multiple items in one step using a High Resolution Camera.
System Design
Number of Reading Devices

Marking and verifying print quality on round packages using 6 image sensors to capture a full 360° view. Three types of verification are available: Presence, Quality, and Reference Check. Existing printers can easily be integrated.
System Design
Number of Reading Devices

Serialization using 6 image sensors to capture a 360° view of any round container such as bottles or vials. The compact design is optimized to fit over existing conveyors for minimal disruption to production lines.
System Design
Number of Reading Devices
Configuration of 6 image sensors achieves 360° view of round packages in-line for aggregation into secondary packaging (boxes, cases, pallets). Eliminates the need for extra equipment to inspect codes typically printed onto lids or caps.
System Design
Number of Reading Devices

Integrates three applications into one compact serialization system. Printing, visual code verification, and secure tamper-evident sealing for compliance in global traceability.
System Design
Number of Reading Devices