 |
Table of Contents:
TA Tip
- Thermal analysis of polymers; Part 3: DSC of thermosets
Applications
- Rapid investigation of thermally hazardous substances
- Thermal analysis of zoledronic acid hydrate
- Determination of safety data for azides using model free kinetics (MFK)
- Thermal analysis of milk powder
- Measurement of the stress-strain behavior of films in the TMA
- Investigation of a hot melt adhesive by TMA
Rapid investigation of thermally hazardous substances
Differential Scanning Calorimetry (DSC) is used to measure the enthalpy of reaction and reaction rates of chemical reactions. This information is important for designing processes, for identifying and assessing potential hazards in chemical syntheses and decomposition reactions, and for thermodynamic and kinetic calculations. Whether a product is thermally unstable or represents a potential explosion hazard (thermal run away) must be determined at a very early stage. DSC and TGA screening measurements using small amounts of substance provide the necessary data for assessing the potential risks.
Introduction
Assessing the potential hazards presented by chemicals and chemical reactions is a never-ending analytical task in the laboratory and in production. An important question concerns the potential danger that could arise through an uncontrolled temperature increase in the handling of materials and their storage.
The risk of a thermal runaway and an explosion with a large amount of damage is significant if
- the reaction enthalpy is large and exothermic,
- the rate of increase of temperature is high or even self-accelerating,
- gaseous products are produced, whether through decomposition or vaporization,
- the reactor system cannot withstand high pressure and/or high temperatures,
- subsequent complications such as fires or environmental pollution are caused.
The potential danger not only has to be estimated with regard to the points mentioned above but also with regard to the probability that such an effect can occur and how large the possible damage would be. In such risk analyses, the determination of the reaction enthalpy of a chemical synthesis or a decomposition reaction is often the starting point for further investigations. The focus is naturally on high risks.
Past experience has however shown that all chemicals and processes must be investigated, not just the ones that are thought to be potentially dangerous. Processes that seem at first sight harmless are synthesis reactions, standard operations such as drying or grinding, and storage.
[…]
Thermal analysis of zoledronic acid hydrate
Zoledronic acid is a drug substance that is used in different pharmaceutical products. This article describes how we investigated the thermal behavior of zoledronic acid hydrate using a METTLER TOLEDO DSC-Microscopy System and a TGA coupled to a mass spectrometer (TGA-MS).
Introduction
Zoledronic acid (or zoledronate) is a drug substance (API) that is used in different pharmaceutical products such as Zometa® or Aclasta® to treat osteoporosis (bone diseases) and bone metastases. Zoledronic acid is a white powder and exists as the anhydride, and mono-, sesqui- and trihydrates. In the Internet, values for the melting point of the anhydride range from 193 °C to 204 °C (www.lookchem.com/Zoledronic-Acid/). The decomposition temperature is given as 239 °C (www.pipharm.com/products/msds/msds-28079.pdf).
This article describes how the thermal behavior of zoledronic acid hydrate was investigated using DSC-Microscopy and TGA-MS.
[…]
Determination of safety data for azides using model free kinetics (MFK)
The enthalpy of decomposition of azides is relatively large. This article shows how DSC can be used to investigate the decomposition kinetics and thermal stability of such substances.
Structure and properties of azides
Azides are salts and organic compounds of hydrazoic acid. Their general structure is shown in Figure 1. R can be a metal ion (e.g. Na+) or even a hydrocarbon chain.
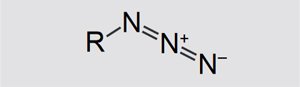 |
Figure 1. Structure of an azide. |
Sodium azide (NaN3) is a typical example of an azide. The substance is used as a propellant in many car airbag systems. The decomposition reaction is triggered by heat or an electrical impulse. The reaction almost instantaneously produces a large volume of nitrogen. A well-known organic azide is azidothymidine, an antiretroviral drug used in the treatment of AIDS. In this study, two organic azides namely C14H19N3O6 (C14) and C20H30N6O14 (C20) were analyzed to determine their thermal behavior and to make predictions about their reactivity.
[…]
Thermal analysis of milk powder
Amorphous lactose is one of the main constituents of milk powder (powdered milk, dried milk) and is highly hygroscopic. If milk powder is stored in an open container, it becomes lumpy due to the uptake of moisture. The lactose present in milk powder can also crystallize as a result of increased water content. This can lead to changes in the flavor and taste of products containing the milk powder. In this article, we show how the behavior of lactose in milk powder can be investigated using TGA-Sorption and DSC measurements.
Introduction
Cow milk consists of 87.5% water, 4.8% carbohydrate (mainly lactose), 3.5% protein (mainly casein), 0.7% trace elements and vitamins as well as about 4.2% fat. If the water is removed, milk powder remains as a residue. There are several different kinds of milk powder. Table 1 presents an overview of the approximate composition of the most important types.
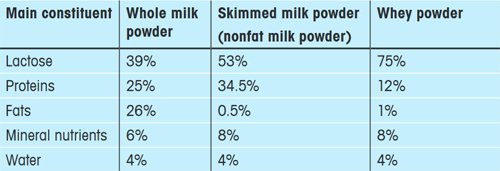 |
Table 1. Overview of three different types of milk powder and their composition (Source: www.agroscope. admin.ch/trockenmilch/01920). |
Due to processing conditions, the lactose present in all kinds of milk powder is in the amorphous state. Milk powders are therefore hygroscopic. The uptake of moisture can lower the glass transition temperature of the lactose to values below ambient temperature. The lactose then softens and the milk powder becomes lumpy.
If the water content is sufficiently high, the lactose can also crystallize. This leads to changes in the taste and consistency of products in which milk powder is used. In this article, we show how the influence of moisture on the glass transition temperature and the crystallization behavior of lactose in milk powder can be investigated by TGA-Sorption and DSC measurements.
[…]
Measurement of the stress-strain behavior of films by TMA
Measurement of the expansion and shrinkage behavior of fibers or films by TMA is generally considered to be rather difficult. In this article, we show that this view is unfounded. Stress-strain measurements can in fact be performed with good reproducibility and provide reliable information about the shrinkage behavior of materials.
Introduction
The shrinkage behavior of fibers or films can be easily investigated by TMA.
In such experiments, the sample is subjected to a constant stress and the sample length measured as a function of temperature. In practice, several questions arise:
- Can the low forces used be calibrated?
- How accurate is the force exerted on the sample?
- How reproducible is the measurement of the shrinkage process?
- How sensitive is the shrinkage behavior to the applied force?
These and other topics will be addressed in this article.
[…]
Investigation of a hot melt adhesive by TMA
Hot melt adhesives, also known as hot glues, are very popular because they are solvent free and very convenient and easy to use. They are often supplied in cylindrical sticks of different diameters, designed to be melted in a hot gun. Chemically, they are based on thermoplastic polymer raw materials such as polyamides or polyurethane and polyester elastomers. Their melting behavior or gel point is of great importance depending on the particular application. This can be determined by means of TMA measurements and is illustrated in the following example.
Introduction
A TMA measurement records the dimensional changes of a sample as a function of time or temperature while it is subjected to a defined force [1]. The force is exerted by a probe (a sensor) that usually rests on the surface of the sample during the measurement. Small forces are used in an experiment to determine the expansion or shrinkage of a sample and appreciably larger forces to record the softening of the sample [2].
In addition, so-called Dynamic Load TMA (DLTMA) measurements can be performed in which the value of the force changes periodically, that is, the force oscillates between a small and large force [3]. The smaller the amplitude of the resulting measurement curve, the lower the elasticity of a sample.
In this article, TMA experiments were performed to characterize a hot melt adhesive using a large force in order to detect the glass transition and the softening and melting ranges. In addition, the DLTMA technique was used to measure the gel point of the sample. Here the gel point is defined as the point at which the sample softens and the probe can finally be pulled out of the previously hard sample to which it had adhered.
Nowadays, many different types of adhesive are in use, for example acrylate adhesives, rubber adhesives and hot melt adhesives to name a few. Hot melt adhesives were originally based on non-adhesive synthetic resins that can be heated and melted at temperatures of up to 180 °C. The glue is soft and tacky when hot but hardens on cooling, thus providing good adhesive properties at room temperature. The melting of the resin at high temperatures led to the name “hot melt”.
Hot melt adhesives have very varied applications. They are used as packaging adhesives, for example in the food industry, as adhesives in the clothing industry, or even for the manufacture of diapers (nappies). Besides being used in stick form, they can also be applied by spraying or dipping.
[…]
References
[1] PET, Physical curing by Dynamic Load TMA, UserCom 5, 15.
[2] Georg Widmann, Interpreting TMA curves, UserCom 14, 1–4.
[3] Determination of curing behavior us - ing TMA/SDTA, UserCom 7, 16–18




