The Characterization of Polymorphs by Thermal Analysis
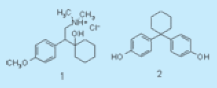 Figure 1. Venlafaxine ((±)-1-[2(dimethylamino)-1-(4-methoxyphenyl)ethyl]cyclohexanol hydrochloride (Structure 1)) and 1,1-bis(4-hydroxyphenyl)cyclohexane (Structure 2).
Figure 1. Venlafaxine ((±)-1-[2(dimethylamino)-1-(4-methoxyphenyl)ethyl]cyclohexanol hydrochloride (Structure 1)) and 1,1-bis(4-hydroxyphenyl)cyclohexane (Structure 2).TGA-FTIR was used to characterize and distinguish between different polymorphs. If two polymorphic forms of a solid are present, whereby one form melts and the other sublimes or vaporizes at about the same temperature, then evolved gas analysis can be used to obtain quantitative (mass loss) and qualitative (spectral) data to analyze such solids. The two pharmaceutically important compounds shown in Figure 1, the active pharmaceutical ingredient (API) venlafaxine hydrochloride (Structure 1) and the well-known host material 1,1-bis (4-hydroxyphenyl)cyclohexane (Structure 2), were analyzed by DSC, TGA, hot-stage microscopy (HSM) and TGA-FTIR to study the phase transitions that occur on heating.
A substance is said to exhibit polymorphism if it can exist in two or more crystal lattice forms. These are called polymorphs and have different physical properties [1].
Venlafaxine Hydrochloride (VenHCl)
VenHCl is a widely sold anti-depressant. The hydrochloride salt of venlafaxine, (±)-1-[2(dimethylamino)-1-(4-methoxy-phenyl)ethyl]cyclohexanol, exists in several different polymorphic modifications. The polymorphs of VenHCl are classified according to their main melting temperatures in the DSC: Form 1 (210-212 °C), Form 2 (208-210 °C), Form 3 (202-204 °C, phase from the melt) and Form 4 (219-220 °C, hydrate/alcoholate). A new amorphous, transient, glassy (semisolid) phase (Form 5) was isolated by sublimation under vacuum during the course of our thermal studies on this API [2].
TGA, TGA-FTIR and DSC Measurements
The TGA curves of the marketed drug Forms 1 and 2 showed complete loss of mass between 220 and 260 °C (Figure 2a). We interpreted the mass loss as being due to decomposition or vaporization of the sample after melting...
Download the full text of this article below.
Conclusions
The phase relationships between VenHCl polymorphs are summarized in Figure 9. In addition to recording quantitative and reproducible data on four previously reported Forms 1 to 4 of VenHCl, a new form, Form 5, was obtained by sublimation. Form 5 is short-lived under inert conditions (stable for a few hours up to one day). It transforms to the hydrate, Form 4, in the open air and to Form 1 under dry conditions.
TGA, DSC and hot-stage microscopy show that the 5 K lower melting solid 2m form of BHPC is the metastable modification and 2s is the thermodynamically stable phase. The single endothermic peak after reheating both forms is ascribed to the thermodynamically stable, higher melting phase, which can also be obtained by vaporization/sublimation.
In addition to the above application, we have also used TGA-FTIR to differentiate between aniline and phenol inclusion in a guest-selective host lattice [4].
The Characterization of Polymorphs by Thermal Analysis | Thermal Analysis Application No. UC253 | Application published in METTLER TOLEDO Thermal Analysis UserCom 25